
[Thu. 17 Nov. 2016] Prof. Seunghoon Shin/Synthetic Methods Development based on Rational Analysis of Intermediates
| Attachment '1' |
|---|
| Speaker | Prof. Seunghoon Shin |
|---|---|
| Date | Thursday 17 November 2016 |
| Time | 5:00pm |
| Venue | #331, Asan Hall, College of Science |
“Synthetic Methods Development based on
Rational Analysis of Intermediates”
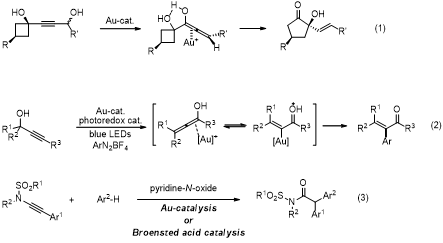
Proper analysis and identification of reaction intermediates in catalysis often lead to new reaction discovery. In this presentation, we describe some of our recent investigations in the gold and Brőnsted acid catalysis. Allenols are found to be transient intermediates in the gold-catalyzed Meyer-Schuster rearrangement of propargyl alcohols en route to enones. In our tandem Meyer-Schuster rearrangement and ring expansion (eq. 1),1 the allenol intermediates play key roles in the diastereo- and enantioselective ring expansion of cyclobutanols having a butyne-1,4-diol unit into cyclopentanones. A related allenol intermediate could also be utilized in the context of cross-coupling chemistry. Compared to the dual Au-photoredox cross-coupling of C(sp3)-Au intermediates,2a the corresponding cross-coupling of C(sp2)-Au species for the formation of C(sp2)- C(sp2) coupling is more challenging, due to a facile proto-demetallation of C(sp2)-Au bond. The Au(I)-Au(III) cycle enabled under the photoredox condition is fast enough to outcompete this undesired side pathway (eq. 2).2b-c This protocol is catalytic both in gold- and photocatalyst and utilizes diazoniums for the dual role of external oxidant and coupling partner. Lastly, oxygenative cross-coupling of alkynes has received tremendous growth, as it obviates the use of diazo precursors. Yet, a concrete support for the involvement of Au-carbene species is still lacking and, especially, intermolecular coupling is very rare. Using Brőnsted acid as a catalytic promotor instead of Au-complex, this transformation occurs under exceptionally mild conditions allowing couplings of diverse nucleophiles (eq. 3). In addition, a SN2’ mechanism could be supported by the formation of scalemic products when chiral N-oxides were used.
References
1. An, J. -H.; Yun, H.; Shin, S.; Shin, S., Adv. Synth. Catal. 2014, 356, 3749.
2. (a) Sahoo, B.; Hopkinson, M. N.; Glorius, F. J. Am. Chem. Soc. 2013, 135, 5505; (b) Patil, D. V.; Yun, H.; Shin, S. Adv. Synth. Catal. 2015, 357, 2622; (c) Um, J.; Yun, H.; Shin, S. Org. Lett. 2016,18, 484.
3. Unpublished results, Patil, D. V.; Kim, S.
Prof. Seunghoon Shin
Department of Chemistry, Hanyang University, South Korea
« Prev [Thu. 1 Dec. 2016] Prof. Sang-Yong Ju/Highly Pure Carbon Nano...
[Thu. 1 Dec. 2016] Prof. Sang-Yong Ju/Highly Pure Carbon Nano... 2016.11.28by 〈[Thu. 3 Nov. 2016] Prof. Min-Sik Kim/Mass spectrometry and it... Next »
[Thu. 3 Nov. 2016] Prof. Min-Sik Kim/Mass spectrometry and it... 2016.10.31by 〉-
Read More
[Thu. 18 May. 2017] Student Host Colloquium : 1,1-Bisborylalkane: New Class of Organoboron Compound for the Chemo, Regio and Stereoselective Organic Transformations
Prof. Seung Hwan ChoThu. 18 May. 2017 -
Read More
[Thu. 11 May. 2017] Prof. Andreas Heinrich/IBS Research, Center for Quantum Nanoscience, Seoul, South Korea
Prof. Andreas HeinrichThu. 11 May. 2017 -
Read More
[Thu. 27 Apr. 2017] Seminar was canceled!
-- -
Read More
[Thu. 13 Apr. 2017] Dr. Wan Ki Bae/Photoelectronic Hybrid Research Center, Korea Institute of Science and Technology, Seoul, Korea
Dr. Wan Ki BaeThu. 13 Apr. 2017 -
Read More
[Thu. 6 Apr. 2017] Prof. Rong-Jie Chein/Institute of Chemistry, Academia Sinica, Nankang, Taipei, Taiwan
Prof. Rong-Jie CheinThu. 6 Apr. 2017 -
Read More
[Thu. 30 Mar. 2017] Prof. Jeong Ho Cho/SKKU Advanced Institute of Nanotechnology (SAINT), School of Chemical Engineering, Sungkyunkwan University, Suwon, Korea
Prof. Jeong Ho ChoThu. 30 Mar. 2017 -
Read More
[Thu. 23 Mar. 2017] Prof. Sangsu Bae/Department of Chemistry, Hanyang University, Seoul, South Korea
Prof. Sangsu BaeThu. 23 Mar. 2017 -
Read More
[Fri. 17 Mar. 2017] 9th RINS Symposium
-Fri. 17 Mar. 2017 -
Read More
[Thu. 9 Mar. 2017] Prof. Woon Ju Song/Department of Chemistry, Seoul National University, Seoul, Korea
Prof. Woon Ju SongThu. 9 Mar. 2017 -
Read More
Spring semester 2017 Seminar Schedule
-- -
Read More
[Mon. 9 Jan. 2017] Prof. Sungwook Woo/Wyss Institute for Biologically Inspired Engineering, Harvard University, USA
Prof. Sungwook WooMon. 9 Jan. 2017 -
Read More
[Wed. 28 Dec. 2016] Prof. James Y. Hamilton/Development of a Broad Range of Asymmetric C–C Bond Forming Reactions with a Versatile Iridium Catalyst
Prof. James Y. HamiltonWednesday 28 December 2016 -
Read More
[Thu. 1 Dec. 2016] Prof. Sang-Yong Ju/Highly Pure Carbon Nanotubes sorted by a Flavin for Thin Film Transistor Applications
Prof. Sang-Yong JuThursday 1 December 2016 -
Read More
[Thu. 17 Nov. 2016] Prof. Seunghoon Shin/Synthetic Methods Development based on Rational Analysis of Intermediates
Prof. Seunghoon ShinThursday 17 November 2016 -
Read More
[Thu. 3 Nov. 2016] Prof. Min-Sik Kim/Mass spectrometry and its applications to Biological Discovery
Prof. Min-Sik KimThursday 3 November 2016 -
Read More
[Thu. 20 Oct. 2016] Prof. Chan Hyuk Kim/Novel Small Molecule-based Approaches for Cancer Immunotherapy
Prof. Chan Hyuk KimThursday 20 October 2016 -
Read More
[Mon. 13 Oct. 2016] Prof. Amir H. Hoveyda/Multicomponent Catalytic Synthesis of Organoboron Compounds for Scalable Natural Product Synthesis
Prof. Amir H. HoveydaMonday 13 October 2016 -
Read More
[Thu. 6 Oct. 2016] Prof. Young S. Park/Atomistic Molecular Engineering of Polycyclic Aromatic Hydrocarbons
Prof. Young S. ParkThursday 6 October 2016 -
Read More
[Thu. 29 Sep. 2016] Prof. Tae-Young Yoon/Observation of Single Membrane Proteins under Mechanical Tension
Prof. Tae-Young YoonThursday 29 September 2016 -
Read More
[Thu. 22 Sep. 2016] Prof. Anna Lee/Organocatalyzed Asymmetric Reactions
Prof. Anna LeeThursday 22 September 2016
Designed by sketchbooks.co.kr / sketchbook5 board skin