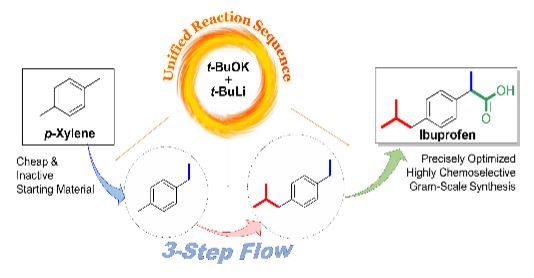
From p-Xylene to Ibuprofen in Flow: 3-Step Synthesis via Unified Sequence of Chemoselective C–H Metalations
| author | Heejin Kim |
|---|---|
| Homepage | https://gowithflow.wixsite.com/hkim |
| journal | Chem. Eur. J. 2019, 25, accepted article. |
Heejin Kim (교신저자)
Ibuprofen was prepared from an inactive and inexpensive p-xylene by 3-step flow functionalizations through chemoselective metalations of benzyl positions in sequence using an in-situ generated LICKOR-type superbase. The flow approach in the microreactor facilitated to comprehensively explore over 100 conditions in the first-step reaction by varying concentrations, temperatures, solvents, and equivalents of reagents, enabling to find the optimal condition with 95% yield by significantly suppressing the formation of byproducts, followed by the second C–H metalation step in 95% yield. Moreover, gram-scale synthesis of ibuprofen in the final step was achieved by biphasic flow reaction of solution-phase intermediate with CO2, isolating 2.3 g for 10 min of operation time.
https://onlinelibrary.wiley.
« Prev Two Different Length-Dependent Regimes in Thermoelectric Larg...
 Two Different Length-Dependent Regimes in Thermoelectric Larg...
2019.08.02by webmaster
〈
Two Different Length-Dependent Regimes in Thermoelectric Larg...
2019.08.02by webmaster
〈
Mid-wavelength Infrared Photoluminescence and Lasing of Tellu... Next »
 Mid-wavelength Infrared Photoluminescence and Lasing of Tellu...
2019.07.22by webmaster
〉
Mid-wavelength Infrared Photoluminescence and Lasing of Tellu...
2019.07.22by webmaster
〉
Articles
-
 Significantly Improved Morphology and Efficiency of Nonhalogenated Solvent-Proces...
Significantly Improved Morphology and Efficiency of Nonhalogenated Solvent-Proces...
-
 Mechanical Force Induces Ylide-Free Cycloaddition of Nonscissible Aziridines
Mechanical Force Induces Ylide-Free Cycloaddition of Nonscissible Aziridines
-
 Bright ligand-activatable fluorescent protein for high-quality multicolor live-ce...
Bright ligand-activatable fluorescent protein for high-quality multicolor live-ce...
-
 Mid-wavelength Infrared Photoluminescence and Lasing of Tellurium Element Solid a...
Mid-wavelength Infrared Photoluminescence and Lasing of Tellurium Element Solid a...
-
 Power Factor of One Molecule Thick Films and Length Dependence
Power Factor of One Molecule Thick Films and Length Dependence
-
 An Emerging Molecular Design Approach to Heavy-Atom-Free Photosensitizers for Enh...
An Emerging Molecular Design Approach to Heavy-Atom-Free Photosensitizers for Enh...
-
 Ga‐Based Liquid Metal Micro/Nanoparticles: Recent Advances and Applications
Ga‐Based Liquid Metal Micro/Nanoparticles: Recent Advances and Applications
-
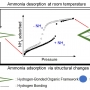 A Hydrogen-Bonded Organic Framework with Type IV NH3 Adsorption Behavior
A Hydrogen-Bonded Organic Framework with Type IV NH3 Adsorption Behavior
-
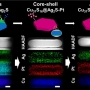 Janus to Core-Shell to Janus: Facile Cation Movement in Cu2-xS/Ag2S Hexagonal Nan...
Janus to Core-Shell to Janus: Facile Cation Movement in Cu2-xS/Ag2S Hexagonal Nan...
-
 Two Different Length-Dependent Regimes in Thermoelectric Large-Area Junctions of ...
Two Different Length-Dependent Regimes in Thermoelectric Large-Area Junctions of ...
-
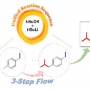 From p-Xylene to Ibuprofen in Flow: 3-Step Synthesis via Unified Sequence of Chem...
From p-Xylene to Ibuprofen in Flow: 3-Step Synthesis via Unified Sequence of Chem...
-
 Mid-wavelength Infrared Photoluminescence and Lasing of Tellurium Element Solid a...
Mid-wavelength Infrared Photoluminescence and Lasing of Tellurium Element Solid a...
-
 Structure–thermopower relationships in molecular thermoelectrics
Structure–thermopower relationships in molecular thermoelectrics
-
 Cytoplasmic Protein Imaging with Mid-Infrared Photothermal Microscopy: Cellular D...
Cytoplasmic Protein Imaging with Mid-Infrared Photothermal Microscopy: Cellular D...
-
 Conjugated Polyelectrolytes as Multifunctional Passivating and Hole‐Transporting ...
Conjugated Polyelectrolytes as Multifunctional Passivating and Hole‐Transporting ...
-
 Covalently Linked Perylene Diimide–Polydiacetylene Nanofibers Display Enhanced St...
Covalently Linked Perylene Diimide–Polydiacetylene Nanofibers Display Enhanced St...
-
 Blue Emission of α-GaN Colloidal Quantum Dots via Zn Doping
Blue Emission of α-GaN Colloidal Quantum Dots via Zn Doping
-
 Gas‐phase conformations of intrinsically disordered proteins and their complexes ...
Gas‐phase conformations of intrinsically disordered proteins and their complexes ...
-
 Multifunctional Self-Doped Nanocrystal Thin-Film Transistor Sensors
Multifunctional Self-Doped Nanocrystal Thin-Film Transistor Sensors
-
 Fine-tuning of wettability in a single metal–organic framework via postcoordinati...
Fine-tuning of wettability in a single metal–organic framework via postcoordinati...
Designed by sketchbooks.co.kr / sketchbook5 board skin