Previous Article Next Article Table of Contents Total Syntheses of Arcyriaflavin A and Calothrixin B Using 2,2′-Bisindole-3-acetic Acid Derivative as a Common Intermediate
| author | Cheol-Hong Cheon |
|---|---|
| Homepage | https://sites.google.com/site/cheonresearchlab/ |
| journal | Org. Lett., 2017, 19 (11), pp 2785–2788 |
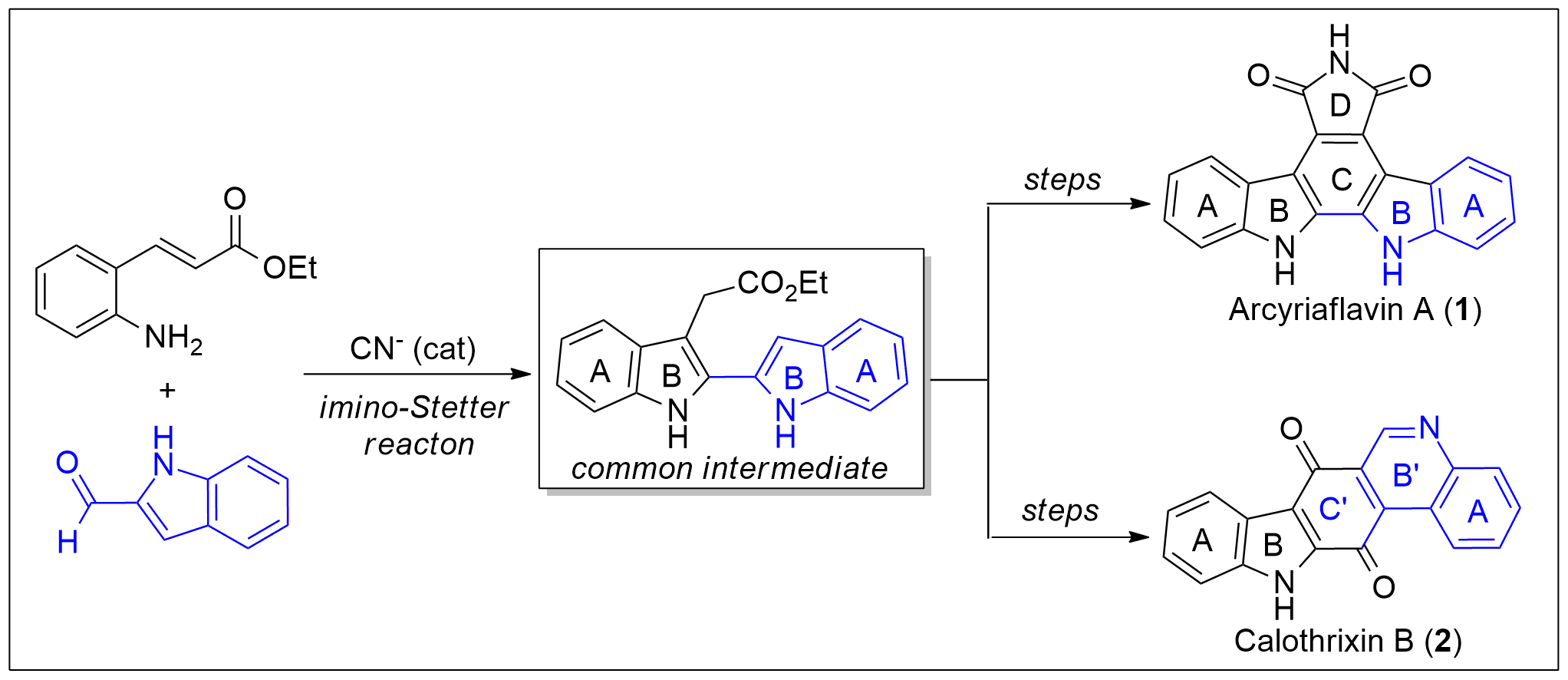
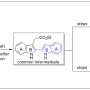
A new protocol for the synthesis of 2,2′-bisindole-3-acetic acid derivatives from aldimines derived from 2-aminocinnamic acid derivatives and indole-2-carboxaldehyde was developed via a cyanide-catalyzed imino-Stetter reaction. With this protocol, the divergent total syntheses of arcyriaflavin A, a representative indolocarbazole natural product, and calothrixin B, a representative indolo[3,2-j]phenanthridine natural product, were completed using a 2,2′-bisindole-3-acetic acid derivative as the common intermediate.
http://pubs.acs.org/doi/full/10.1021/acs.orglett.7b00687
« Prev Collision Cross Sections and Ion Structures: Development of a...
 Collision Cross Sections and Ion Structures: Development of a...
2017.10.13by Manager
〈
Collision Cross Sections and Ion Structures: Development of a...
2017.10.13by Manager
〈
Synthesis, Characterization, and Efficient Catalytic Activiti... Next »
 Synthesis, Characterization, and Efficient Catalytic Activiti...
2017.09.26by Manager
〉
Synthesis, Characterization, and Efficient Catalytic Activiti...
2017.09.26by Manager
〉
Articles
-
 Molecular Role of Ca2+ and Hard Divalent Metal Cations on Accelerated Fibrillatio...
Molecular Role of Ca2+ and Hard Divalent Metal Cations on Accelerated Fibrillatio...
-
 Supramolecular Modulation of Structural Polymorphism in Pathogenic α-Synuclein Fi...
Supramolecular Modulation of Structural Polymorphism in Pathogenic α-Synuclein Fi...
-
 Recent Progress in the Chemistry of Pyridazinones for Functional Group Transforma...
Recent Progress in the Chemistry of Pyridazinones for Functional Group Transforma...
-
 Maskless Arbitrary Writing of Molecular Tunnel Junctions
Maskless Arbitrary Writing of Molecular Tunnel Junctions
-
 Single Component Organic Solar Cells Based on Oligothiophene-Fullerene Conjugate
Single Component Organic Solar Cells Based on Oligothiophene-Fullerene Conjugate
-
 Lanthanide metal-assisted synthesis of rhombic dodecahedral MNi (M=Ir and Pt) nan...
Lanthanide metal-assisted synthesis of rhombic dodecahedral MNi (M=Ir and Pt) nan...
-
 Radially Phase Segregated PtCu@PtCuNi Dendrite@Frame Nanocatalyst for the Oxygen ...
Radially Phase Segregated PtCu@PtCuNi Dendrite@Frame Nanocatalyst for the Oxygen ...
-
 Collision Cross Sections and Ion Structures: Development of a General Calculation...
Collision Cross Sections and Ion Structures: Development of a General Calculation...
-
 Previous Article Next Article Table of Contents Total Syntheses of Arcyriaflavin ...
Previous Article Next Article Table of Contents Total Syntheses of Arcyriaflavin ...
-
 Synthesis, Characterization, and Efficient Catalytic Activities of a Nickel(II) P...
Synthesis, Characterization, and Efficient Catalytic Activities of a Nickel(II) P...
-
 Molecular Insights into Human Serum Albumin as a Receptor of Amyloid-β in the Ext...
Molecular Insights into Human Serum Albumin as a Receptor of Amyloid-β in the Ext...
-
 Two Regioisomeric π-Conjugated Small Molecules: Synthesis, Photophysical, Packing...
Two Regioisomeric π-Conjugated Small Molecules: Synthesis, Photophysical, Packing...
-
 Major Electronic Transition Shift from Bandgap to Localized Surface Plasmon Reson...
Major Electronic Transition Shift from Bandgap to Localized Surface Plasmon Reson...
-
 Rational Design of In Vivo Tau Tangle-Selective Near Infrared Fluorophores: Expan...
Rational Design of In Vivo Tau Tangle-Selective Near Infrared Fluorophores: Expan...
-
 Achieving Highly Efficient Nonfullerene Organic Solar Cells with Improved Intermo...
Achieving Highly Efficient Nonfullerene Organic Solar Cells with Improved Intermo...
-
 Ionic effect on the excited-state proton transfer reactions in aqueous solutions
Ionic effect on the excited-state proton transfer reactions in aqueous solutions
-
 A conductive porous organic polymer with superprotonic conductivity of a Nafion-t...
A conductive porous organic polymer with superprotonic conductivity of a Nafion-t...
-
 Quantum optical measurements with undetected photons through vacuum field indisti...
Quantum optical measurements with undetected photons through vacuum field indisti...
-
 Nanoscale Control of Amyloid Self-Assembly Using Protein Phase Transfer by Host-G...
Nanoscale Control of Amyloid Self-Assembly Using Protein Phase Transfer by Host-G...
-
 A Mitochondria-targeted Cryptocyanine-Based Photothermogenic Photosensitizer
A Mitochondria-targeted Cryptocyanine-Based Photothermogenic Photosensitizer
Designed by sketchbooks.co.kr / sketchbook5 board skin