May 4, 2023 (Thursday) Professor Seungyoon Hong
| Speaker | Professor Seungyoon Hong (Department of Chemistry, Seoul National University) |
|---|---|
| Date | Thursday, May 4, 2023 |
| Time | 05:00 PM |
| Venue | Asan Science Hall 331 |
Navigating the Periodic Table for Catalysis:
From Transition Metal to Main Group Elements
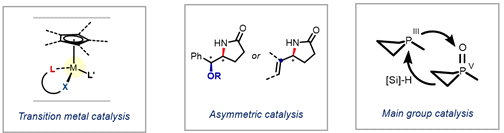
Catalytic reactions that construct carbon-nitrogen (C−N) bonds are of sustained interest in the synthetic organic community since they can offer a streamlined route to useful nitrogen-containing motifs commonly encountered in natural products and clinical drugs.1 The prevailing strategy to meet such demands in industrial settings is currently shaped by transition metal-catalyzed methods, as exemplified by Buchwald-Hartwig aminations. Despite the significant strides in this area, increasing emphasis has been placed on developing a cost-effective and sustainable alternative that omits a transition metal and repositions the main group elements from the catalytic periphery to the center of bond making and breaking during catalysis.
In this talk, I will first describe research efforts aimed at inventing new transition-metal catalysts, enabling mild and efficient C−N bond-forming reactions via the metal-nitrenoid transfer pathway.2-4 This mechanistic framework ushered in the further development of chiral variants, revealing new opportunities in asymmetric synthesis. Second, a functionally and mechanistically distinct main group-catalyzed method will be presented.5 By accentuating the biphilic reactivity of phosphacycles, we are able to use nitroalkanes—many of which are stable and inexpensive—as primary aminating reagents for C−N coupling chemistry. This method represents a sustainable way of preparing nitrogen-containing products that not only shows complementarity in scope and selectivity to existing methods but bypasses commonly faced challenges (e.g. overamination and metal contamination) in transition metal catalysis.
References
1. S. Y. Hong, Y. Hwang, M. Lee and S. Chang*, Mechanism-Guided Development of Transition-Metal Catalyzed C–N Bond-Forming Reactions Using Dioxazolones as the Versatile Amidating Source, Acc. Chem. Res. 2021, 54, 2683–2700.
2. S. Y. Hong,† Y. Park,† Y. Hwang, Y. Kim, M.-H. Baik,* and S. Chang* Selective formation of γ-lactams via C–H amidation enabled by tailored iridium catalysts, Science, 2018, 359, 1016-1021. (†Equally contributed)
3. S. Kim, D. Kim, S. Y. Hong* and S. Chang*, Tuning Orbital Symmetry of Iridium Nitrenoid Enables Catalytic Diastereo- and Enantioselective Alkene Difunctionalizations, J. Am. Chem. Soc,, 2021, 143, 10, 3993–4004. (*Co-corresponding authors)
4. S. Y. Hong, D. Kim and S. Chang*, Catalytic Access to Carbocation Intermediates via Nitrenoid Transfer Leading to Allylic Lactams, Nat. Catal., 2021, 4, 79−88.
5. S. Y. Hong and A. T. Radosevich*, Chemoselective Primary Amination of Aryl Boronic Acids by PIII/PV=O Catalysis: Synthetic Capture of the Transient Nef Intermediate HNO, J. Am. Chem. Soc., 2022, 144, 8902–8907.
Articles
- June 8, 2023 (Thursday) Professor Myungki Park
- June 1, 2023 (Thursday) Professor Go Min-seop
- May 25, 2023 (Thu) Professor Hoi-Ri Moon
- May 11, 2023 (Thu) Dr. Changyeol Lee
- May 16, 2023 (Tuesday) Professor Simone Fabiano
- May 16, 2023 (Tuesday) Frontliner Invitational Seminar
- May 11, 2023 (Thu) Professor Hyeonseop Lim
-
 May 4, 2023 (Thursday) Professor Seungyoon Hong
May 4, 2023 (Thursday) Professor Seungyoon Hong
- April 21, 2023 (Fri) Professor Bangsun Dong
- April 13, 2023 (Thu) Professor Baek Yoon-jeong
- April 6, 2023 (Thursday) Professor Hyungmin Kim
- April 6, 2023 (Thursday) Professor Hyungmin Kim
- March 30, 2023 (Thursday) Professor Taesoo Choi
- March 23, 2023 (Thu) Professor Lee Min-jae
- 2023 Germany-Korea On-Site Plenary Discussion on Computational Electrochemistry
- February 24 (Fri) Lawrence Yoon Suk Lee
- December 15th (Thu) Professor Changhyeok Choi
- December 8th (Thu) Dr. Jieon Kwon (KIST)
- December 1st (Thu) Professor Park Yun-soo
- 2022 International Symposium on Chemical Applications of Machine Learning
Designed by sketchbooks.co.kr / sketchbook5 board skin