Directing the surface atomic geometry on copper sulfide for enhanced electrochemical nitogen reduction
| author | Kwangyeol Lee |
|---|---|
| Homepage | http://nanolab.korea.ac.kr/ |
| journal | ACS Catalysis |
Understanding catalytic-conversion determinants will blueprint an efficient electrocatalyst design for electrochemical nitrogen reduction. In metal chalcogenide-based catalysts, metal-site nitrogen adsorption initiates nitrogen fixation, and successive hydrogen supply from nearby chalcogen sites hydrogenates the nitrogen to ammonia. However, surface geometry-dependent reaction kinetics are rarely studied because the reaction is very fast. Here, we investigate the relationship between catalyst geometrical features and their electrochemical nitrogen reduction kinetics using surface atomic geometry-regulated copper sulfide (Cu1.81S) nanocatalysts with exposed (100)- and (010)-type facets for flat and zigzag planes, respectively. The exposed facet densities of the nanocatalysts are varied via their aspect ratios. Nanocrystals with highly exposed (010)-type surfaces exhibit the best nitrogen reduction kinetics. Density functional theory calculation reveals that the protruded Cu and S atomic arrangement on the zigzag (010)-type surface promotes N2 adsorption and facilitates proton transfer from near the S site to *N2 at the Cu site, thus fast-forwarding electrochemical nitrogen reduction.

https://pubs.acs.org/doi/10.1021/acscatal.2c03680?cookieSet=1
« Prev Flattening bent Janus nanodiscs expands lattice parameters
 Flattening bent Janus nanodiscs expands lattice parameters
2023.05.08by webmaster2
〈
Flattening bent Janus nanodiscs expands lattice parameters
2023.05.08by webmaster2
〈
High Seebeck Coefficient Achieved by Multinuclear Organometal... Next »
 High Seebeck Coefficient Achieved by Multinuclear Organometal...
2023.05.08by webmaster2
〉
High Seebeck Coefficient Achieved by Multinuclear Organometal...
2023.05.08by webmaster2
〉
Articles
- Self-Aggregating Tau Fragments Recapitulate Pathologic Phenotypes and Neurotoxici...
-
 Ultranarrow Mid-infrared Quantum Plasmon Resonance of Self-Doped Silver Selenide ...
Ultranarrow Mid-infrared Quantum Plasmon Resonance of Self-Doped Silver Selenide ...
-
 Molecular Thermoelectricity in EGaIn-Based Molecular Junctions
Molecular Thermoelectricity in EGaIn-Based Molecular Junctions
-
 Perovskite Nanocatalsts Protected by Hermetically Sealing for Highly Bright and S...
Perovskite Nanocatalsts Protected by Hermetically Sealing for Highly Bright and S...
-
 The importance of a charge transfer descriptor for screening potential CO2 reduct...
The importance of a charge transfer descriptor for screening potential CO2 reduct...
-
 Resonant Raman-Active Polymer Dot Barcodes for Multiplex Cell Mapping
Resonant Raman-Active Polymer Dot Barcodes for Multiplex Cell Mapping
-
 Decoding the Roles of Amyloid-β (1-42)'s Key Oligomerization Domains toward Desig...
Decoding the Roles of Amyloid-β (1-42)'s Key Oligomerization Domains toward Desig...
-
 Flattening bent Janus nanodiscs expands lattice parameters
Flattening bent Janus nanodiscs expands lattice parameters
-
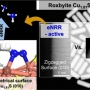 Directing the surface atomic geometry on copper sulfide for enhanced electrochemi...
Directing the surface atomic geometry on copper sulfide for enhanced electrochemi...
-
 High Seebeck Coefficient Achieved by Multinuclear Organometallic Molecular Junctions
High Seebeck Coefficient Achieved by Multinuclear Organometallic Molecular Junctions
-
 Thermopower in Transition from Tunneling to Hopping
Thermopower in Transition from Tunneling to Hopping
-
 An Activity-Based Fluorescent Probe for Imaging Fluctuations of Peroxynitrite (ON...
An Activity-Based Fluorescent Probe for Imaging Fluctuations of Peroxynitrite (ON...
-
 Deep learning for development of organic optoelectronic devices: Efficient prescr...
Deep learning for development of organic optoelectronic devices: Efficient prescr...
-
 Photocatalytic Superoxide Radical Generator that Induces Pytoptosis in Cancer Cells
Photocatalytic Superoxide Radical Generator that Induces Pytoptosis in Cancer Cells
-
 Direct C-H metallation of tetrahydrofuran and application in flow
Direct C-H metallation of tetrahydrofuran and application in flow
-
 Functionalization of Diamine-Appended MOF-Based Adsorbents by Ring Opening of Epo...
Functionalization of Diamine-Appended MOF-Based Adsorbents by Ring Opening of Epo...
-
 High Gravimetric and Volumetric Ammonia Capacities in Robust Metal-Organic Framew...
High Gravimetric and Volumetric Ammonia Capacities in Robust Metal-Organic Framew...
-
 Li-ion Intercalation, Rectification, and Solid Electrolyte Interphase in Molecula...
Li-ion Intercalation, Rectification, and Solid Electrolyte Interphase in Molecula...
-
 Thermopower of Molecular Junction in Harsh Thermal Environments
Thermopower of Molecular Junction in Harsh Thermal Environments
-
 Microfluidics-Assisted Synthesis of Hierarchical Cu2O Nanocrystal as C2-Selective...
Microfluidics-Assisted Synthesis of Hierarchical Cu2O Nanocrystal as C2-Selective...
Designed by sketchbooks.co.kr / sketchbook5 board skin