Adsorption of Carbon Dioxide on Unsaturated Metal Sites in M2(dobpdc) Frameworks with Exceptional Structural Stability and Relation between Lewis Acidity and Adsorption Enthalpy
| author | Chang Seop Hong |
|---|---|
| Homepage | http://immlab.korea.ac.kr/ |
| journal | Chem. Eur. J. 2016, 22,7444 –7451 |
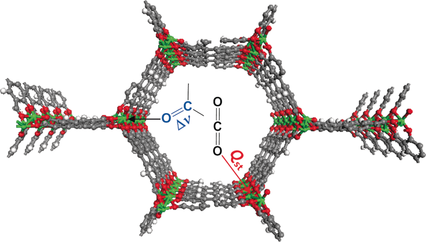
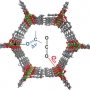
A series of metal–organic frameworks (MOFs) M2(dobpdc) (M=Mn, Co, Ni, Zn; H4dobpdc=4,4′-dihydroxy-1,1′-biphenyl-3,3′-dicarboxylic acid), with a highly dense arrangement of open metal sites along hexagonal channels were prepared by microwave-assisted or simple solvothermal reactions. The activated materials were structurally expanded when guest molecules including CO2 were introduced into the pores. The Lewis acidity of the open metal sites varied in the order Mn<Co<Ni>Zn, as confirmed by C=O stretching bands in the IR spectra, which are related to the CO2 adsorption enthalpy. DFT calculations revealed that the high CO2 binding affinity of transition-metal-based M2(dobpdc) is primarily attributable to the favorable charge transfer from CO2(oxygen lone pair acting as a Lewis base) to the open metal sites (Lewis acid), while electrostatic effects, the underlying factor responsible for the particular order of binding strength observed across different transition metals, also play a role. The framework stability against water coincides with the order of Lewis acidity. In this series of MOFs, the structural stability of Ni2(dobpdc) is exceptional; it endured in water vapor, liquid water, and in refluxing water for one month, and the solid remained intact on exposure to solutions of pH 2–13. The DFT calculations also support the experimental finding that Ni2(dobpdc) has higher chemical stability than the other frameworks.
http://onlinelibrary.wiley.com/doi/10.1002/chem.201600189/full
« Prev Investigation of Charge Carrier Behavior in High Performance ...
 Investigation of Charge Carrier Behavior in High Performance ...
2016.07.29by Manager
〈
Investigation of Charge Carrier Behavior in High Performance ...
2016.07.29by Manager
〈
Programmed activation of cancer cell apoptosis: A tumor-targe... Next »
 Programmed activation of cancer cell apoptosis: A tumor-targe...
2016.07.29by Manager
〉
Programmed activation of cancer cell apoptosis: A tumor-targe...
2016.07.29by Manager
〉
Articles
-
 Highly Efficient Fullerene-Free Polymer Solar Cells Fabricated with Polythiophene...
Highly Efficient Fullerene-Free Polymer Solar Cells Fabricated with Polythiophene...
-
 Low-voltage organic devices based on pristine and self-assembled monolayer-treate...
Low-voltage organic devices based on pristine and self-assembled monolayer-treate...
-
 Higher Quantum State Transitions in Colloidal Quantum Dot with Heavy Electron Doping
Higher Quantum State Transitions in Colloidal Quantum Dot with Heavy Electron Doping
-
 High-harmonic generation: Drive round the twist
High-harmonic generation: Drive round the twist
-
 Gradients of Rectification: Tuning Molecular Electronic Devices by the Controlled...
Gradients of Rectification: Tuning Molecular Electronic Devices by the Controlled...
-
 Investigation of Charge Carrier Behavior in High Performance Ternary Blend Polyme...
Investigation of Charge Carrier Behavior in High Performance Ternary Blend Polyme...
-
 Adsorption of Carbon Dioxide on Unsaturated Metal Sites in M2(dobpdc) Frameworks ...
Adsorption of Carbon Dioxide on Unsaturated Metal Sites in M2(dobpdc) Frameworks ...
-
 Programmed activation of cancer cell apoptosis: A tumor-targeted phototherapeutic...
Programmed activation of cancer cell apoptosis: A tumor-targeted phototherapeutic...
-
 Probing Distinct Fullerene Formation Processes from Carbon Precursors of Differen...
Probing Distinct Fullerene Formation Processes from Carbon Precursors of Differen...
-
 Manifesting Subtle Differences of Neutral Hydrophilic Guest Isomers in a Molecula...
Manifesting Subtle Differences of Neutral Hydrophilic Guest Isomers in a Molecula...
Designed by sketchbooks.co.kr / sketchbook5 board skin