Synthesis, Characterization, and Efficient Catalytic Activities of a Nickel(II) Porphyrin: Remarkable Solvent and Substrate Effects on Participation of Multiple Active Oxidants
| author | Suk Joong Lee |
|---|---|
| Homepage | http://inmlab.korea.ac.kr/ |
| journal | Chem. Eur. J. 2017, 23, 11969 – 11976(DOI: 10.1002/chem.201702750) |
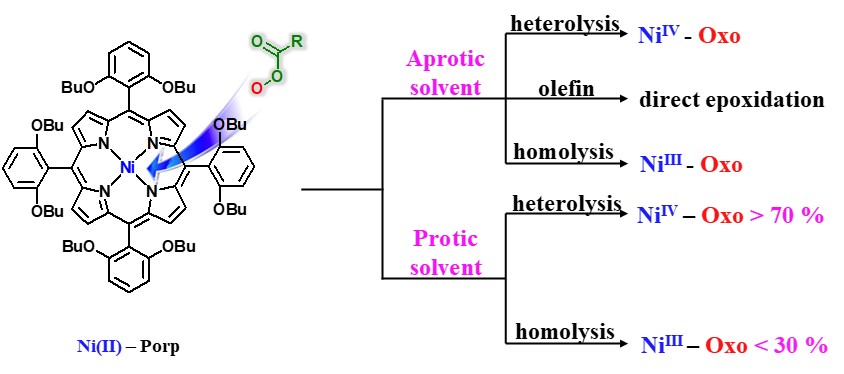
A new nickel(II) porphyrin complex, [NiII(porp)] (1), has been synthesized and characterized by 1H NMR, 13C NMR and mass spectrometry analysis. This NiII porphyrin complex 1 quantitatively catalyzed the epoxidation reaction of a wide range of olefins with meta-chloroperoxybenzoic acid (m-CPBA) under mild conditions. Reactivity and Hammett studies, H218O-exchange experiments, and the use of PPAA (peroxyphenylacetic acid) as a mechanistic probe suggested that participation of multiple active oxidants NiII−OOC(O)R 2, NiIV-Oxo 3, and NiIII-Oxo 4 within olefin epoxidation reactions by the nickel porphyrin complex is markedly affected by solvent polarity, concentration, and type of substrate. In aprotic solvent systems, such as toluene, CH2Cl2, and CH3CN, multiple oxidants, NiII−(O)R 2, NiIV-Oxo 3, and NiIII-Oxo 4, operate simultaneously as the key active intermediates responsible for epoxidation reactions of easy-to-oxidize substrate cyclohexene, whereas NiIV-Oxo 3 and NiIII-Oxo 4 species become the common reactive oxidant for the difficult-to-oxidize substrate 1-octene. In a protic solvent system, a mixture of CH3CN and H2O (95:5), the NiII−OOC(O)R 2 undergoes heterolytic or homolytic O−O bond cleavage to afford NiIV-Oxo 3and NiIII-Oxo 4 species by general acid catalysis prior to direct interaction between 2 and olefin, regardless of the type of substrate. In this case, only NiIV-Oxo 3 and NiIII-Oxo 4 species were the common reactive oxidant responsible for olefin epoxidation reactions.
« Prev Previous Article Next Article Table of Contents Total Synthes...
 Previous Article Next Article Table of Contents Total Synthes...
2017.10.10by Manager
〈
Previous Article Next Article Table of Contents Total Synthes...
2017.10.10by Manager
〈
Molecular Insights into Human Serum Albumin as a Receptor of ... Next »
 Molecular Insights into Human Serum Albumin as a Receptor of ...
2017.09.25by Manager
〉
Molecular Insights into Human Serum Albumin as a Receptor of ...
2017.09.25by Manager
〉
Articles
-
 Molecular Role of Ca2+ and Hard Divalent Metal Cations on Accelerated Fibrillatio...
Molecular Role of Ca2+ and Hard Divalent Metal Cations on Accelerated Fibrillatio...
-
 Supramolecular Modulation of Structural Polymorphism in Pathogenic α-Synuclein Fi...
Supramolecular Modulation of Structural Polymorphism in Pathogenic α-Synuclein Fi...
-
 Recent Progress in the Chemistry of Pyridazinones for Functional Group Transforma...
Recent Progress in the Chemistry of Pyridazinones for Functional Group Transforma...
-
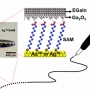 Maskless Arbitrary Writing of Molecular Tunnel Junctions
Maskless Arbitrary Writing of Molecular Tunnel Junctions
-
 Single Component Organic Solar Cells Based on Oligothiophene-Fullerene Conjugate
Single Component Organic Solar Cells Based on Oligothiophene-Fullerene Conjugate
-
 Lanthanide metal-assisted synthesis of rhombic dodecahedral MNi (M=Ir and Pt) nan...
Lanthanide metal-assisted synthesis of rhombic dodecahedral MNi (M=Ir and Pt) nan...
-
 Radially Phase Segregated PtCu@PtCuNi Dendrite@Frame Nanocatalyst for the Oxygen ...
Radially Phase Segregated PtCu@PtCuNi Dendrite@Frame Nanocatalyst for the Oxygen ...
-
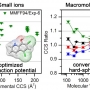 Collision Cross Sections and Ion Structures: Development of a General Calculation...
Collision Cross Sections and Ion Structures: Development of a General Calculation...
-
 Previous Article Next Article Table of Contents Total Syntheses of Arcyriaflavin ...
Previous Article Next Article Table of Contents Total Syntheses of Arcyriaflavin ...
-
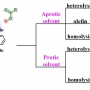 Synthesis, Characterization, and Efficient Catalytic Activities of a Nickel(II) P...
Synthesis, Characterization, and Efficient Catalytic Activities of a Nickel(II) P...
-
 Molecular Insights into Human Serum Albumin as a Receptor of Amyloid-β in the Ext...
Molecular Insights into Human Serum Albumin as a Receptor of Amyloid-β in the Ext...
-
 Two Regioisomeric π-Conjugated Small Molecules: Synthesis, Photophysical, Packing...
Two Regioisomeric π-Conjugated Small Molecules: Synthesis, Photophysical, Packing...
-
 Major Electronic Transition Shift from Bandgap to Localized Surface Plasmon Reson...
Major Electronic Transition Shift from Bandgap to Localized Surface Plasmon Reson...
-
 Rational Design of In Vivo Tau Tangle-Selective Near Infrared Fluorophores: Expan...
Rational Design of In Vivo Tau Tangle-Selective Near Infrared Fluorophores: Expan...
-
 Achieving Highly Efficient Nonfullerene Organic Solar Cells with Improved Intermo...
Achieving Highly Efficient Nonfullerene Organic Solar Cells with Improved Intermo...
-
 Ionic effect on the excited-state proton transfer reactions in aqueous solutions
Ionic effect on the excited-state proton transfer reactions in aqueous solutions
-
 A conductive porous organic polymer with superprotonic conductivity of a Nafion-t...
A conductive porous organic polymer with superprotonic conductivity of a Nafion-t...
-
 Quantum optical measurements with undetected photons through vacuum field indisti...
Quantum optical measurements with undetected photons through vacuum field indisti...
-
 Nanoscale Control of Amyloid Self-Assembly Using Protein Phase Transfer by Host-G...
Nanoscale Control of Amyloid Self-Assembly Using Protein Phase Transfer by Host-G...
-
 A Mitochondria-targeted Cryptocyanine-Based Photothermogenic Photosensitizer
A Mitochondria-targeted Cryptocyanine-Based Photothermogenic Photosensitizer
Designed by sketchbooks.co.kr / sketchbook5 board skin