
Nanometric Water Channels in Water-in-Salt Lithium Ion Battery Electrolyte
| author | Kyungwon Kwak and Minhaeng Cho |
|---|---|
| Homepage | https://cmsd.ibs.re.kr/html/cmsd_en/ |
| journal | J. Am. Chem. Soc., Article ASAP DOI: 10.1021/jacs.8b07696 |
| Attachment '2' |
|---|

Joonhyung Lim(박사과정)
Lithium-ion batteries (LIBs) have been deployed in a wide range of energy-storage applications and helped to revolutionize technological development. Recently, a lithium ion battery that uses superconcentrated salt water as its electrolyte has been developed. However, the role of water in facilitating fast ion transport in such highly concentrated electrolyte solutions is not fully understood yet. Here, femtosecond IR spectroscopy and molecular dynamics simulations are used to show that bulk-like water coexists with interfacial water on ion aggregates. We found that dissolved ions form intricate three-dimensional ion−ion networks that are spontaneously intertwined with nanometric water hydrogen-bonding networks. Then, hydrated lithium ions move through bulk-like water channels acting like conducting wires for lithium ion transport. Our experimental and simulation results indicate that water structure-breaking chaotropic anion salts with a high propensity to form ion networks in aqueous solutions would be excellent candidates for water-based LIB electrolytes. We anticipate that the present work will provide guiding principles for developing aqueous LIB electrolytes.

https://pubs.acs.org/doi/abs/10.1021/jacs.8b07696
« Prev A New Approach for Large-Area Thermoelectric Junctions with L...
 A New Approach for Large-Area Thermoelectric Junctions with L...
2018.11.13by webmaster
〈
A New Approach for Large-Area Thermoelectric Junctions with L...
2018.11.13by webmaster
〈
Topotactic Transformations in an Icosahedral Nanocrystal to F... Next »
 Topotactic Transformations in an Icosahedral Nanocrystal to F...
2018.11.12by webmaster
〉
Topotactic Transformations in an Icosahedral Nanocrystal to F...
2018.11.12by webmaster
〉
-
Read More
Harnessing Intramolecular Rotation to Enhance Two‐photon Imaging of Aβ Plaques Through Minimizing Background Fluorescence
Jong Seung Kimhttp://orgchem.korea.ac.krJinwoo Shin(제1저자 석박통합과정) The aggregation of Aβ‐proteins in senile plaques is a critical event during the development of Alzheimer’s disease, and the postmortem detection of Aβ‐rich proteinaceous deposits via fluores...Date2019.03.18 Bywebmaster Views1263 -
Read More

Proteogenomic Characterization of Human Early-Onset Gastric Cancer
Sang-Won Leehttp://ionchem.korea.ac.kr/Dong-Gi Mun(제1저자, 박사과정) We report proteogenomic analysis of diffuse gastric cancers (GCs) in young populations. Phosphoproteome data elucidated signaling pathways associated with somatic mutations based on mutation-phosphorylation co...Date2019.01.21 Bywebmaster Views799 -
Read More
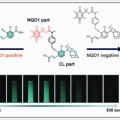
Chemiluminescent Probe for the In Vitro and In Vivo Imaging of Cancers Over-Expressing NQO1
Jong Seung Kimhttp://orgchem.korea.ac.krActivatable (turn-on) probes that permit the rapid, sensitive, selective, and accurate identification of cancer-associated biomarkers can help drive advances in cancer research. Herein, a NAD(P)H:quinone oxidoreductase- 1 (NQO1)-specific ch...Date2019.01.11 Bywebmaster Views1475 -
Read More
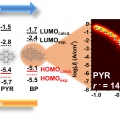
Molecularly Controlled Stark Effect Induces Significant Rectification in Polycyclic Aromatic Hydrocarbon-terminated n-Alkanethiolates
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlSoo Jin Cho(제1저자, 석박통합과정) Gyu Don Kong(공동 제1저자, 박사과정) Variation of electronic structure of individual molecules as a function of applied bias matters for performance of molecular and organic electronic devices. Understandi...Date2018.12.26 Bywebmaster Views1060 -
Read More
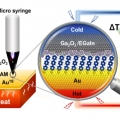
A New Approach for Large-Area Thermoelectric Junctions with Liquid Eutectic Gallium-Indium Electrode
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlSohyun Park(통합과정) A challenge in organic thermoelectrics is to relate thermoelectric performance of devices to the chemical and electronic structures of organic component inside them on a molecular scale. To this end, a reliable and rep...Date2018.11.13 Bywebmaster Views2214 -
Read More

Nanometric Water Channels in Water-in-Salt Lithium Ion Battery Electrolyte
Kyungwon Kwak and Minhaeng Chohttps://cmsd.ibs.re.kr/html/cmsd_en/Joonhyung Lim(박사과정) Lithium-ion batteries (LIBs) have been deployed in a wide range of energy-storage applications and helped to revolutionize technological development. Recently, a lithium ion battery that uses superconcentrated salt w...Date2018.11.12 Bywebmaster Views948 -
Read More

Topotactic Transformations in an Icosahedral Nanocrystal to Form Efficient Water-Splitting Catalysts
Kwangyeol Leehttp://nanolab.korea.ac.kr/Designing high‐performance, precious‐metal‐based, and economic electrocatalysts remains an important challenge in proton exchange membrane (PEM) electrolyzers. Here, a highly active and durable bifunctional electrocatalyst for PEM electroly...Date2018.11.12 Bywebmaster Views1985 -
Read More
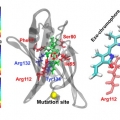
Fluorescence enhancement of a ligand-activated fluorescent protein induced by collective noncovalent interactions
Sang-Hee Shim and Minhaeng Chohttps://cmsd.ibs.re.kr/html/cmsd_en/Euihyun Lee(박사과정) Fluorescent proteins contain an internal chromophore constituted of amino acids or an external chromophore covalently bonded to the protein. To increase their fluorescence intensities, many research groups have attempt...Date2018.11.12 Bywebmaster Views1090 -
Read More
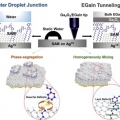
Elucidating the Role of Molecule-Electrode Interfacial Defects in Charge Tunneling Characteristics of Large-area Junctions
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlGyu Don Kong(박사과정) Junji Jin(석사과정) Interfacial chemistry at organic-inorganic contact critically determines function of a wide range of molecular and organic electronic devices and other systems. The chemistry is, however, difficult...Date2018.11.12 Bywebmaster Views881 -
Read More
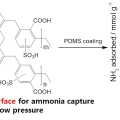
PDMS-Coated Hypercrosslinked Porous Organic Polymers Modified via Double Postsynthetic Acidifications for Ammonia Capture
Chang Seop Honghttps://www.immlab.korea.ac.kr/Dong Won Kang(통합과정) Minjung Kang(통합과정) A hypercrosslinked porous organic polymer was modified by post-oxidation and post-sulfonation to obtain a porous platform with high density of acidic groups. Such acidified material exhibits re...Date2018.11.12 Bywebmaster Views11302 -
Read More
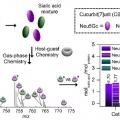
Accurate Quantification of N-glycolylneuraminic Acid in Therapeutic Proteins Using Supramolecular Mass Spectrometry
Hugh I. Kimhttps://sites.google.com/site/hughkimgroup/Hyun Hee Lee (박사과정) Chae Eun Heo (박사과정) Practical applications of innovative host-guest systems are challenging because of unexpected guest competitors and/or subtle environmental differences. Herein, a supramolecular mass spectrome...Date2018.11.12 Bywebmaster Views845 -
Read More
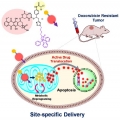
Overcoming Drug Resistance by Targeting Cancer Bioenergetics with an Activatable Prodrug
Jong Seung Kimhttp://orgchem.korea.ac.kr/Nearly without exception, all known cancer chemotherapeutics elicit a resistance response over time. The resulting resistance is correlated with poor clinical outcomes. Here we report a new approach to overcoming resistance that involves re...Date2018.11.12 Bywebmaster Views961 -
Read More
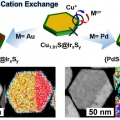
Janus Nanoparticle Structural Motif Control via Asymmetric Cation Exchange in Edge-Protected Cu1.81S@IrxSy Hexagonal Nanoplates
Kwangyeol Leehttp://nanolab.korea.ac.kr/Post-synthetic transformation of nanoparticles has received great attention, because this approach can provide an unusual route to elaborately composition-controlled nanostructures while maintaining the overall structure of the template. In...Date2018.11.12 Bywebmaster Views1496 -
Read More

Deconvolution of Tunneling Current in Large-area Junctions Formed with Mixed Self-Assembled Monolayers
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlJunji Jin(석사과정) Gyu Don Kong(박사과정) Whereas single-component self-assembled monolayers (SAMs) have served widely as organic components in molecular and organic electronics, how the performance of the device is influenced by the hetero...Date2018.11.12 Bywebmaster Views924 -
Read More
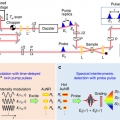
Electron heating and thermal relaxation of gold nanorods revealed by two-dimensional electronic spectroscopy
Minhaeng Chohttp://cmsd.ibs.re.kr/html/cmsd_en/To elucidate the complex interplay between the size and shape of gold nanorods and their electronic, photothermal, and optical properties for molecular imaging, photothermal therapy, and optoelectronic devices, it is a prerequisite to chara...Date2018.11.12 Bywebmaster Views1110 -
Read More
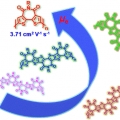
(Semi)ladder-Type Bithiophene Imide-Based All-Acceptor Semiconductors: Synthesis, Structure–Property Correlations, and Unipolar n-Type Transistor Performance
Han Young Woohttp://www.ooml.korea.ac.kr/Development of high-performance unipolar n-type organic semiconductors still remains as a great challenge. In this work, all-acceptor bithiophene imide-based ladder-type small molecules BTIn and semiladder-type homopolymers PBTIn (n = 1&nda...Date2018.11.12 Bywebmaster Views1022 -
Read More
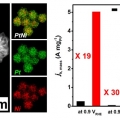
Dendrite-Embedded Platinum–Nickel Multiframes as Highly Active and Durable Electrocatalyst toward the Oxygen Reduction Reaction
Kwangyeol Leehttp://nanolab.korea.ac.kr/Hyukbu Kwon (석사과정) Jongsik Park (박사과정) Pt-based nanoframe catalysts have been explored extensively due to their superior activity toward the oxygen reduction reaction (ORR). Herein, we report the synthesis of Pt–Ni multiframes...Date2018.11.12 Bywebmaster Views910 -
Read More
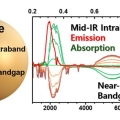
Mid-Infrared Intraband Transition of Metal Excess Colloidal Ag2Se Nanocrystals
Kwang Seob Jeonghttps://kwangsjeong.wixsite.com/ksjlab-koreaunivMihyeon Park (석사과정) Dongsun Choi (박사과정) Steady-state intraband transition, which is a promising electronic transition of a colloidal quantum dot along with the band-gap transition, had been a long-standing challenge. The steady-stat...Date2018.11.12 Bywebmaster Views1378 -
Read More
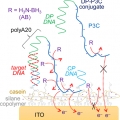
Enhanced Electron Transfer Mediated by Conjugated Polyelectrolyte and Its Application to Washing-Free DNA Detection
Han Young Woohttp://Journal of the American Chemical Society. 2017. Article ASAP (DOI: 10.1021/jacs.7b12382)Ji-Eun Jeong (박사과정) Direct electron transfer between a redox label and an electrode requires a very short working distance (<1-2 nm), and in general an affinity biosensor based on the direct electron transfer requires a finely smoothed ...Date2018.02.20 ByManager Views1851 -
Read More
Vertex-reinforced PtCuCo ternary nanoframes as efficient and stable electrocatalysts for the oxygen reduction reaction and the methanol oxidation reaction
Kwangyeol Leehttp://nanolab.korea.ac.kr/Taehyun Kwon(박사과정) / Minki Jun (박사과정) Noble metal binary alloy nanoframes have emerged as a new class of fuel cell electrocatalysts because of their intrinsic high catalytic surface area and accompanied high catalytic activity. Howe...Date2018.02.20 ByManager Views1960
Designed by sketchbooks.co.kr / sketchbook5 board skin