
Bright ligand-activatable fluorescent protein for high-quality multicolor live-cell super-resolution microscopy
| author | Sang-Hee Shim |
|---|---|
| Homepage | https://cmsd.ibs.re.kr/html/cmsd_en/ |
| journal | nature communications |
| Attachment '1' |
|---|
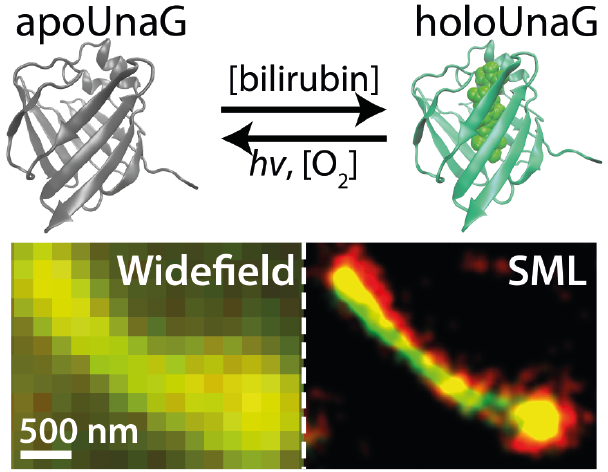
We introduce UnaG as a green-to-dark photoswitching fluorescent protein capable of high-quality super-resolution imaging with photon numbers equivalent to the brightest photoswitchable red protein. UnaG only fluoresces upon binding of a fluorogenic metabolite, bilirubin, enabling UV-free reversible photoswitching with easily controllable kinetics and low background under Epi illumination. The on- and off-switching rates are controlled by the concentration of the ligand and the excitation light intensity, respectively, where the dissolved oxygen also promotes the off-switching. The photo-oxidation reaction mechanism of bilirubin in UnaG suggests that the lack of ligand-protein covalent bond allows the oxidized ligand to detach from the protein, emptying the binding cavity for rebinding to a fresh ligand molecule. We demonstrate super-resolution single-molecule localization imaging of various subcellular structures genetically encoded with UnaG, which enables facile labeling and simultaneous multicolor imaging of live cells. UnaG has the promise of becoming a default protein for high-performance super-resolution imaging.
https://www.nature.com/
« Prev Mechanical Force Induces Ylide-Free Cycloaddition of Nonsciss...
 Mechanical Force Induces Ylide-Free Cycloaddition of Nonsciss...
2020.01.17by webmaster
〈
Mechanical Force Induces Ylide-Free Cycloaddition of Nonsciss...
2020.01.17by webmaster
〈
Mid-wavelength Infrared Photoluminescence and Lasing of Tellu... Next »
 Mid-wavelength Infrared Photoluminescence and Lasing of Tellu...
2020.01.17by webmaster
〉
Mid-wavelength Infrared Photoluminescence and Lasing of Tellu...
2020.01.17by webmaster
〉
-
Read More
Significantly Improved Morphology and Efficiency of Nonhalogenated Solvent-Processed Solar Cells Derived from a Conjugated Donot-Accepteor Block Copolymer
Dong Hoon Choihttp://fpl.korea.ac.kr/A highly crystalline conjugated donor (D)–acceptor (A) block copolymer (PBDT2T‐b ‐N2200) that has good solubility in nonhalogenated solvents is successfully synthesized. PBDT2T‐b ‐N2200 shows a broad complementary absorption behavior ...Date2023.05.03 Bywebmaster2 Views426 -
Read More
Mechanical Force Induces Ylide-Free Cycloaddition of Nonscissible Aziridines
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlSangmin Jung Reported herein is that aziridines can be a nonvulnerable mechanophore. Upon exposure to a mechanical force, stereochemically pure nonactivated aziridines incorporated into the backbone of macromolecule do not undergo cis‐trans...Date2020.01.17 Bywebmaster Views797 -
Read More
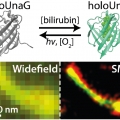
Bright ligand-activatable fluorescent protein for high-quality multicolor live-cell super-resolution microscopy
Sang-Hee Shimhttps://cmsd.ibs.re.kr/html/cmsd_en/We introduce UnaG as a green-to-dark photoswitching fluorescent protein capable of high-quality super-resolution imaging with photon numbers equivalent to the brightest photoswitchable red protein. UnaG only fluoresces upon binding of a fluo...Date2020.01.17 Bywebmaster Views810 -
Read More
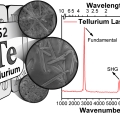
Mid-wavelength Infrared Photoluminescence and Lasing of Tellurium Element Solid and Microcrystals
Kwang Seob Jeonghttps://kwangsjeong.wixsite.com/ksjlab-koreaunivDongsun Choi(제1저자, 통합과정) Tellurium has been of great interest in physics, chemistry, material science, and more recently in nanoscience. However, information on the photoluminescence of Te crystals, crucial in understanding the mater...Date2020.01.17 Bywebmaster Views1053 -
Read More
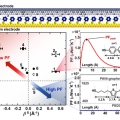
Power Factor of One Molecule Thick Films and Length Dependence
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlSohyun Park(제1저자, 통합과정) Seohyun Kang(공동 제1저자, 통합과정) There is a rapidly increasing interest in organic thin film thermoelectrics. However, the power factor of one molecule thick organic film, the self-assembled monolayer (SAM...Date2019.12.06 Bywebmaster Views662 -
Read More
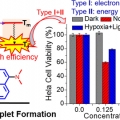
An Emerging Molecular Design Approach to Heavy-Atom-Free Photosensitizers for Enhanced Photodynamic Therapy under Hypoxia
Sungnam Parkhttps://ultrafastspec.wixsite.com/sparkSangin Kim(제1저자, 통합과정생) A novel strategy for designing highly efficient and activatable photosensitizers that can effectively generate reactive oxygen species (ROS) under both normoxia and hypoxia is proposed. Replacing both oxygen ...Date2019.10.10 Bywebmaster Views601 -
Read More
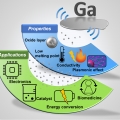
Ga‐Based Liquid Metal Micro/Nanoparticles: Recent Advances and Applications
Kwangyeol Lee, Hyo Jae Yoonhttp://nanolab.korea.ac.kr/Hyunsun Song(지도교수: 윤효재), Taekyung Kim (지도교수: 이광렬), Seohyun Kang (지도교수: 윤효재), Haneul Jin (지도교수: 이광렬) / 공동 제 1저자 Liquid metals are emerging as fluidic inorganic materials in various research fields. Micro‐ and...Date2019.10.07 Bywebmaster Views898 -
Read More
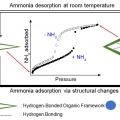
A Hydrogen-Bonded Organic Framework with Type IV NH3 Adsorption Behavior
Chang Seop Honghttps://www.immlab.korea.ac.kr/Dong Won Kang(강동원, 통합과정, 제1저자) / Minjung Kang(강민정, 통합과정, 제2저자) An S‐shaped gas isotherm pattern displays high working capacity in pressure‐swing adsorption cycle, as established for CO2, CH4, acetylene, and CO. However, ...Date2019.10.07 Bywebmaster Views8408 -
Read More
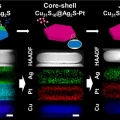
Janus to Core-Shell to Janus: Facile Cation Movement in Cu2-xS/Ag2S Hexagonal Nanoplates Induced by Surface Strain Control
Kwangyeol Leehttp://nanolab.korea.ac.kr/Taekyung Kim, Jongsik Park, Yongju Hong(공동 제 1저자) Nanocrystals with multiple compositions and hetero-interfaces have received a great attention due to promising multifunctional and synergistic physicochemical properties. In particular,...Date2019.10.02 Bywebmaster Views1088 -
Read More
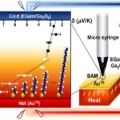
Two Different Length-Dependent Regimes in Thermoelectric Large-Area Junctions of n-Alkanethiolates
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlSohyun Park(제1저자, 통합과정) Molecular thermoelectrics is relatively unexplored compared with its analogous research field, molecular electronics. This is surprising considering that the two research fields share an identical energy lands...Date2019.08.02 Bywebmaster Views977 -
Read More
From p-Xylene to Ibuprofen in Flow: 3-Step Synthesis via Unified Sequence of Chemoselective C–H Metalations
Heejin Kimhttps://gowithflow.wixsite.com/hkimHeejin Kim (교신저자) Ibuprofen was prepared from an inactive and inexpensive p-xylene by 3-step flow functionalizations through chemoselective metalations of benzyl positions in sequence using an in-situ generated LICKOR-type superbase. Th...Date2019.07.26 Bywebmaster Views993 -
Read More
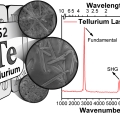
Mid-wavelength Infrared Photoluminescence and Lasing of Tellurium Element Solid and Microcrystals
Kwang Seob Jeonghttps://kwangsjeong.wixsite.com/ksjlab-koreaunivDongsun Choi(제1저자, 통합과정) Tellurium has been of great interest in physics, chemistry, material science, and more recently in nanoscience. However, information on the photoluminescence of Te crystals, crucial in understanding the mater...Date2019.07.22 Bywebmaster Views754 -
Read More
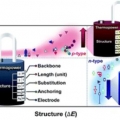
Structure–thermopower relationships in molecular thermoelectrics
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlSohyun Park (통합과정, 제1저자) Hungu Kang(Post-Doc. 제1저자) Studies of molecular thermoelectrics help to reveal how atomic-detailed structural modification in molecules can affect the thermopower of molecular-scale devices. This review co...Date2019.06.07 Bywebmaster Views560 -
Read More
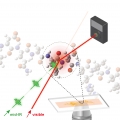
Cytoplasmic Protein Imaging with Mid-Infrared Photothermal Microscopy: Cellular Dynamics of Live Neurons and Oligodendrocytes
Minhaeng Chohttps://cmsd.ibs.re.kr/html/cmsd_en/Chanjong Park(통합과정, 제1저자) Mid-infrared photothermal microscopy has been suggested as an alternative to conventional infrared microscopy because in addition to the inherent chemical contrast available upon vibrational excitation, it c...Date2019.06.07 Bywebmaster Views2613 -
Read More

Conjugated Polyelectrolytes as Multifunctional Passivating and Hole‐Transporting Layers for Efficient Perovskite Light‐Emitting Diodes
Han Young Woohttps://www.ooml.korea.ac.kr/Thanh Luan Nguyen(Post-Doc. 제1저자) Metal halide perovskites (MHPs) have attracted significant attention as light‐emitting materials owing to their high color purities and tunabilities. A key issue in perovskite light‐emitting diodes (PeLE...Date2019.06.07 Bywebmaster Views906 -
Read More
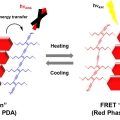
Covalently Linked Perylene Diimide–Polydiacetylene Nanofibers Display Enhanced Stability and Photocurrent with Reversible FRET Phenomenon
Sungnam Parkhttps://ultrafastspec.wixsite.com/sparkBecause of their unique structural and optical properties, 1D perylene diimide (PDI) derivatives have gained attention for use in optoelectronic devices. However, PDI‐containing self‐assembled supramolecular systems often are of limited use ...Date2019.06.07 Bywebmaster Views1856 -
Read More
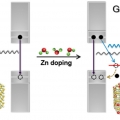
Blue Emission of α-GaN Colloidal Quantum Dots via Zn Doping
Kwang Seob Jeonghttps://kwangsjeong.wixsite.com/ksjlab-koreaunivYun Chang Choi(최윤창, 제1저자) Gallium nitride (GaN) has been of interest due to its enormous potentials for optoelectronic devices. However, it is very interesting that no proper bottom-up chemical synthesis method for GaN quantum dot has...Date2019.05.27 Bywebmaster Views757 -
Read More
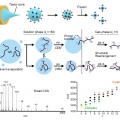
Gas‐phase conformations of intrinsically disordered proteins and their complexes with ligands: Kinetically trapped states during transfer from solution to the gas phase
Hugh I. Kimhttps://www.hughkimlab.com(Jong Yoon Han, 제1저자, 박사과정) Flexible structures of intrinsically disordered proteins (IDPs) are crucial for versatile functions in living organisms, which involve interaction with diverse partners. Electrospray ionization ion mobilit...Date2019.05.27 Bywebmaster Views2238 -
Read More
Multifunctional Self-Doped Nanocrystal Thin-Film Transistor Sensors
Kwang Seob Jeonghttps://kwangsjeong.wixsite.com/ksjlab-koreaunivDongsun Choi(제1저자, 통합과정) Self-doping in nanocrystals allows accessing higher quantum states. The electrons occupying the lowest energy state of the conduction band form a metastable state that is very sensitive to the electrostatic p...Date2019.03.18 Bywebmaster Views741 -
Read More

Fine-tuning of wettability in a single metal–organic framework via postcoordination modification and its reduced graphene oxide aerogel for oil–water separation
Chang Seop Honghttps://www.immlab.korea.ac.kr/Sunhwi Eom(제1저자) Elaborate control of wettability in a single platform is essential for materials’ applications towards oil–water separation, but it still remains challenging. Herein, we performed postcoordination modificatio...Date2019.03.18 Bywebmaster Views999
Designed by sketchbooks.co.kr / sketchbook5 board skin