
Kinetic Modulation of Amyloid-β (1-42) Aggegation and Toxicity by Structure-Based Rational Design
| author | Hugh I. Kim |
|---|---|
| Homepage | https://www.hughkimlab.com/ |
| journal | Journal of the American Chemical Society |
Several point mutations can modulate protein structure and dynamics, leading to different natures. Especially in the case of amyloidogenic proteins closely related to neurodegenerative diseases, structural changes originating from point mutations can affect fibrillation kinetics. Herein, we rationally designed mutant candidates to inhibit the fibrillation process of amyloid-β with its point mutants through multistep in silico analyses. Our results showed that the designed mutants induced kinetic self-assembly suppression and reduced the toxicity of the aggregate. A multidisciplinary biophysical approach with small-angle X-ray scattering, ion mobility-mass spectrometry, mass spectrometry, and additional in silico experiments was performed to reveal the structural basis associated with the inhibition of fibril formation. The structure-based design of the mutants with suppressed self-assembly performed in this study could provide a different perspective for modulating amyloid aggregation based on the structural understanding of the intrinsically disordered proteins.

https://pubs.acs.org/doi/10.1021/jacs.1c10173

Lim Dongjun
(First author, integrated course student)
-
Read More

Chemical Fields: Directing Atom Migration in the Multiphasic Nanocrystal
Kwangyeol Leehttp://nanolab.korea.ac.kr/Atoms in a bulk solid phase are usually trapped to fixed positions and can change their position only under certain conditions (e.g., at a melting point) due to the high energy barrier of migration between positions within the crystal lattic...Date2023.05.08 Bywebmaster2 Views1034 -
Read More
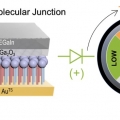
Electronic Mechanism of In Situ Inversion of Rectification Polarity in Supramolecular Engineered Monalayer
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlThis paper describes polarity inversion in molecular rectification and the related mechanism. Using supramolecular engineered, ultrastable binary mixed self-assembled monolayer (SAM) composed of organic molecular diode (SC11BIPY) and inert r...Date2023.05.08 Bywebmaster2 Views419 -
Read More
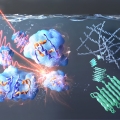
Direct observation of protein structural transitions through entire amyloid aggregation processes in water using 2D-IR spectroscopy
Hugh I. Kim, Kyungwon Kwakhttps://kkwaklab.wixsite.com/quantspeclabAmyloid proteins that undergo self-assembly to form insoluble fibrillar aggregates have attracted much attention due to their role in biological and pathological significance in amyloidosis. This study aims to understand the amyloid aggregat...Date2023.05.08 Bywebmaster2 Views366 -
Read More
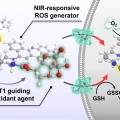
Harnessing GLUT1-Targeted Pro-oxidant Ascorbate for Synergistic Phototherapeutics
Jong Seung Kimhttp://orgchem.korea.ac.krDespite extensive efforts to realize effective photodynamic therapy (PDT), there is still a lack of therapeutic approaches concisely structured to mitigate the major obstacles of PDT in clinical applications. Herein, we report a molecular st...Date2023.05.08 Bywebmaster2 Views270 -
Read More
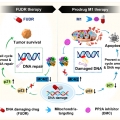
DNA-Damage-Response-Targeting Mitochondri-Activated Multifunctional Prodrug Strategy for Self-Denfensive Tumor Therapy
Jong Seung Kimhttp://orgchem.korea.ac.kr/We report a novel multifunctional construct, M1, designed explicitly to target the DNA damage response in cancer cells. M1 contains both a floxuridine (FUDR) and protein phosphatase 2A (PP2A) inhibitor combined with a GSH-sensitive linker. F...Date2023.05.04 Bywebmaster2 Views8942 -
Read More
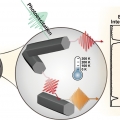
Midwavelength Infrared Colloidal Nanowire Laser
Kwang Seob Jeonghttps://kwangsjeong.wixsite.com/ksjlab-koreaunivRealizing bright colloidal infrared emitters in the midwavelength infrared (or mid-IR), which can be used for low-power IR light-emitting diodes (LEDs), sensors, and deep-tissue imaging, has been a challenge for the last few decades. Here, w...Date2023.05.04 Bywebmaster2 Views492 -
Read More
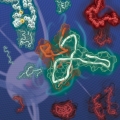
Kinetic Modulation of Amyloid-β (1-42) Aggegation and Toxicity by Structure-Based Rational Design
Hugh I. Kimhttps://www.hughkimlab.com/Several point mutations can modulate protein structure and dynamics, leading to different natures. Especially in the case of amyloidogenic proteins closely related to neurodegenerative diseases, structural changes originating from point muta...Date2023.05.04 Bywebmaster2 Views1019 -
Read More
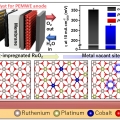
Safeguarding the RuO2 phase against lattice oxygen oxidation during acidic water electrooxidation
Kwangyeol Leehttp://nanolab.korea.ac.kr/Defective RuO2 possesses excellent initial activity toward the oxygen evolution reaction in acidic water electrooxidation due to the involvement of lattice oxygens, which, however, is the very reason for the accelerated dissolution of Ru spe...Date2023.05.04 Bywebmaster2 Views620 -
Read More
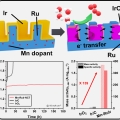
Mn-dopant differentiating the Ru and Ir oxidation states in catalytic oxides toward durable oxygen evolution reaction in acidic electrolyte
Kwangyeol Leehttp://nanolab.korea.ac.kr/Designing an efficient and durable electrocatalyst for the sluggish oxygen evolution reaction (OER) at the anode remains the foremost challenge in developing proton exchange membrane (PEM) electrolyzers. Here we report a highly active and du...Date2023.05.04 Bywebmaster2 Views932 -
Read More

Dynamic Water Promotes Lithium-Ion Transport in Superconcentrated and Eutectic Aqueous Electrolytes
Kyungwon Kwak, Minhaeng Chohttps://kkwaklab.wixsite.com/quantspeclabSuperconcentrated aqueous electrolytes have shown promise as safe and high-voltage lithium-ion battery (LIB) electrolytes. However, the interplay of lithium-ion solvation structure and dynamics with fast Li-ion transport has not been elucida...Date2023.05.04 Bywebmaster2 Views597 -
Read More
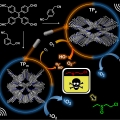
Isomeric sp2-C conjugated Porous Organic Polymer-mediated Photo- and Sono-catalytic Detoxification of Sulfur Mustard Simulant under Ambient Conditions
Jong Seung Kim, Chang Seop Honghttp://MatterThe development of efficient strategies for the sustainable detoxification of mustard gas simulants has longstanding demand for human safety. Here, we present for the first time a photo- and sono-catalyzed selective detoxification of mustard...Date2023.05.04 Bywebmaster2 Views1614 -
Read More

Vertical-crystalline Fe-doped β-Ni oxyhydroxides for highly active and stable oxygen evolution reaction
Kwangyeol Leehttp://nanolab.korea.ac.kr/The layered transition metal oxyhydroxides have received increasing interest owing to the efficient energy conversion performance and material stability during the oxygen evolution reaction (OER). In particular, Fe-doped NiOOH has shown reco...Date2023.05.04 Bywebmaster2 Views538 -
Read More
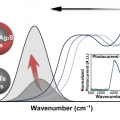
Extended Short-Wavelength Infrared Photoluminescence and Photocurrent of Nonstoichiometric Silver Telluride Collodial Nanocrystals
Kwang Seob Jeonghttps://kwangsjeong.wixsite.com/ksjlab-koreaunivDemands on nontoxic nanomaterials in the short-wavelength infrared (SWIR) have rapidly grown over the past decade. Here, we present the nonstoichiometric silver chalcogenide nanocrystals of AgxTe (x > 2) and Ag2Te/Ag2S CQDs with a tunable ba...Date2023.05.04 Bywebmaster2 Views1029 -
Read More
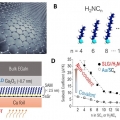
Enhanced Thermopower of Saturated Molecules by Noncovalent Anchor-Induced Electron Doping of Single-Layer Graphene Electrode
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlEnhancing thermopower is a key goal in organic and molecular thermoelectrics. Herein, it is shown that introducing noncovalent contact with a single-layer graphene (SLG) electrode improves the thermopower of saturated molecules as compared t...Date2023.05.04 Bywebmaster2 Views429 -
Read More
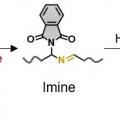
Mechanical Force for the Transformation of Aziridine into Imine
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlForce-selective mechanochemical reactions may be important for applications in polymer mechanochemistry, yet it is difficult to achieve such reactions. This paper reports that cis-N-phthalimidoaziridine incorporated into a macromolecular bac...Date2023.05.04 Bywebmaster2 Views483 -
Read More
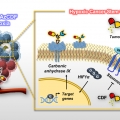
A Small Molecule Strategy for Targeting Cancer Stem Cells in Hypoxic Microenvironments and Preventing Tumorigenesis
Jong Seung Kimhttp://orgchem.korea.ac.kr/Breast cancer consists of heterogenic subpopulations, which determine the prognosis and response to chemotherapy. Among these subpopulations, a very limited number of cancer cells are particularly problematic. These cells, known as breast ca...Date2023.05.04 Bywebmaster2 Views344 -
Read More
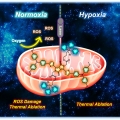
Mitochondria-targeted nanotheranostic: Harnessing single-laser-activated dual phototherapeutic processing for hypoxic tumor treatment
Jong Seung Kimhttp://orgchem.korea.ac.kr/Realizing maximum tumor suppression along with preventing tumor regrowth by optimizing the photon usage in phototherapy remains a major challenge. Herein, a mitochondria-targeted phototheranostic nanoformulation (MsPDTT NPs) was prepared fro...Date2023.05.04 Bywebmaster2 Views390 -
Read More
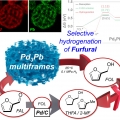
Pb3Pb Nanosponges for Selective Conversion of Furfural to Furfuryl Alcohol under Mild Condition
Heejin Kim, Kwangyeol Leehttp://nanolab.korea.ac.kr/Alloy structures with high catalytic surface areas and tunable surface energies can lead to high catalytic selectivity and activities. Herein, the synthesis of sponge-like Pd3Pb multiframes (Pd3Pb MFs) is reported by using the thermodynamica...Date2023.05.04 Bywebmaster2 Views419 -
Read More

Deep Learning Optical Spectroscopy Based on Experimental Database: Potential Applications to Molecular Design
Sungnam Parkhttps://ultrafastspec.wixsite.com/sparkAccurate and reliable prediction of the optical and photophysical properties of organic compounds is important in various research fields. Here, we developed deep learning (DL) optical spectroscopy using a DL model and experimental database ...Date2023.05.04 Bywebmaster2 Views300 -
Read More

Wettability of graphene and interfacial water structure
Kyungwon Kwak, Minhaeng Chohttps://cmsd.ibs.re.kr/html/cmsd_en/Understanding the wettability of graphene is important for various applications, such as water desalination, energy storage, and catalysis. However, the detailed water hydrogen-bonding structure at the water-graphene interface is not yet ful...Date2023.05.04 Bywebmaster2 Views1115
Designed by sketchbooks.co.kr / sketchbook5 board skin
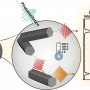 Midwavelength Infrared Colloidal Nanowire Laser
Midwavelength Infrared Colloidal Nanowire Laser
 Safeguarding the RuO2 phase against lattice oxygen oxidation ...
Safeguarding the RuO2 phase against lattice oxygen oxidation ...