
Decoding the Roles of Amyloid-β (1-42)'s Key Oligomerization Domains toward Designing Epitope-Specific Aggregation Inhibitors
| author | Hugh I. Kim |
|---|---|
| Homepage | https://www.hughkimlab.com/ |
| journal | JACS Au |
Fibrillar amyloid aggregates are the pathological hallmarks of multiple neurodegenerative diseases. The amyloid-β (1-42) protein, in particular, is a major component of senile plaques in the brains of patients with Alzheimer’s disease and a primary target for disease treatment. Determining the essential domains of amyloid-β (1-42) that facilitate its oligomerization is critical for the development of aggregation inhibitors as potential therapeutic agents. In this study, we identified three key hydrophobic sites (17LVF19, 32IGL34, and 41IA42) on amyloid-β (1-42) and investigated their involvement in the self-assembly process of the protein. Based on these findings, we designed candidate inhibitor peptides of amyloid-β (1-42) aggregation. Using the designed peptides, we characterized the roles of the three hydrophobic regions during amyloid-β (1-42) fibrillar aggregation and monitored the consequent effects on its aggregation property and structural conversion. Furthermore, we used an amyloid-β (1-42) double point mutant (I41N/A42N) to examine the interactions between the two C-terminal end residues with the two hydrophobic regions, and their roles in amyloid self-assembly. Our results indicate that interchain interactions in the central hydrophobic region (17LVF19) of amyloid-β (1-42) are important for fibrillar aggregation, and its interaction with other domains is associated with the accessibility of the central hydrophobic region for initiating the oligomerization process. Our study provides mechanistic insights into the self-assembly of amyloid-β (1-42) and highlights key structural domains that facilitate this process. Our results can be further applied toward improving the rational design of candidate amyloid-β (1-42) aggregation inhibitors.

https://pubs.acs.org/doi/10.1021/jacsau.2c00668
« Prev Resonant Raman-Active Polymer Dot Barcodes for Multiplex Cell...
 Resonant Raman-Active Polymer Dot Barcodes for Multiplex Cell...
2023.05.08by webmaster2
〈
Resonant Raman-Active Polymer Dot Barcodes for Multiplex Cell...
2023.05.08by webmaster2
〈
Flattening bent Janus nanodiscs expands lattice parameters Next »
 Flattening bent Janus nanodiscs expands lattice parameters
2023.05.08by webmaster2
〉
Flattening bent Janus nanodiscs expands lattice parameters
2023.05.08by webmaster2
〉
-
Read MoreNo Image
Self-Aggregating Tau Fragments Recapitulate Pathologic Phenotypes and Neurotoxicity of Alzheimer's Disease in Mice
Hugh I. Kimhttps://www.hughkimlab.com/In tauopathy conditions, such as Alzheimer's disease (AD), highly soluble and natively unfolded tau polymerizes into an insoluble filament; however, the mechanistic details of this process remain unclear. In the brains of AD patients, o...Date2023.12.20 Bywebmaster2 Views1824 -
Read More

Ultranarrow Mid-infrared Quantum Plasmon Resonance of Self-Doped Silver Selenide Nanocrystal
Kwang Seob Jeonghttps://kwangsjeong.wixsite.com/ksjlab-koreaunivThe infrared quantum plasmon resonance (IR QPR) of nanocrystals (NCs) exhibits the combined properties of classical and quantum mechanics, potentially enabling unprecedented optics. However, research on the development of localized surface ...Date2023.12.20 Bywebmaster2 Views2160 -
Read More

Molecular Thermoelectricity in EGaIn-Based Molecular Junctions
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlUnderstanding the thermoelectric effects that convert energy between heat and electricity on a molecular scale is of great interest to the nanoscience community. As electronic devices continue to be miniaturized to nanometer scales, thermor...Date2023.12.20 Bywebmaster2 Views2068 -
Read More
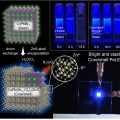
Perovskite Nanocatalsts Protected by Hermetically Sealing for Highly Bright and Stable Deep-Blue Light-Emitting Diodes
Dong Hoon Choi, Kwangyeol Leehttp://nanolab.korea.ac.kr/Metal–halide perovskite nanocrystals (NCs) have emerged as suitablelight-emitting materials for light-emitting diodes (LEDs) and other practicalapplications. However, LEDs with perovskite NCs undergoenvironment-induced and ion-migratio...Date2023.07.04 Bywebmaster2 Views610 -
Read More
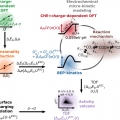
The importance of a charge transfer descriptor for screening potential CO2 reduction on electrocatalysts
Stefan Ringehttps://ringelab.com/It has been over twenty years since the linear scaling of reaction intermediate adsorption energies started to coin the fields of heterogeneous and electrocatalysis as a blessing and a curse at the same time. It has established the possibil...Date2023.07.04 Bywebmaster2 Views901 -
Read More
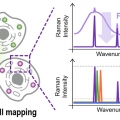
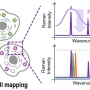
Resonant Raman-Active Polymer Dot Barcodes for Multiplex Cell Mapping
Sang-Hee Shim, Han Young Woohttps://www.ooml.korea.ac.kr/Resonance Raman spectroscopy is an efficient tool for multiplex imaging because of the narrow bandwidth of the electronically enhanced vibrational signals. However, Raman signals are often overwhelmed by concurrent fluorescence. In this stu...Date2023.05.08 Bywebmaster2 Views681 -
Read More
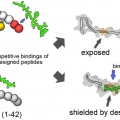
Decoding the Roles of Amyloid-β (1-42)'s Key Oligomerization Domains toward Designing Epitope-Specific Aggregation Inhibitors
Hugh I. Kimhttps://www.hughkimlab.com/Fibrillar amyloid aggregates are the pathological hallmarks of multiple neurodegenerative diseases. The amyloid-β (1-42) protein, in particular, is a major component of senile plaques in the brains of patients with Alzheimer’s dis...Date2023.05.08 Bywebmaster2 Views2791 -
Read More

Flattening bent Janus nanodiscs expands lattice parameters
Kwangyeol Leehttp://ChemNanoscale lattice parameter engineering is a potentially powerful tool for tailoring the electronic properties of nanomaterials. The nascent strain in juxtaposed hetero-interfaces of nanocrystals was recently shown to substantially affect th...Date2023.05.08 Bywebmaster2 Views508 -
Read More
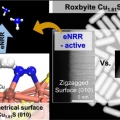
Directing the surface atomic geometry on copper sulfide for enhanced electrochemical nitogen reduction
Kwangyeol Leehttp://nanolab.korea.ac.kr/Understanding catalytic-conversion determinants will blueprint an efficient electrocatalyst design for electrochemical nitrogen reduction. In metal chalcogenide-based catalysts, metal-site nitrogen adsorption initiates nitrogen fixation, and...Date2023.05.08 Bywebmaster2 Views747 -
Read More
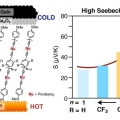
High Seebeck Coefficient Achieved by Multinuclear Organometallic Molecular Junctions
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlThis paper describes thermoelectric property of molecular junctions incorporating multinuclear ruthenium alkynyl complexes that comprise Ru(dppe)2 (dppe = 1,2-bis(diphenylphosphino)ethane) fragments and diethylnyl aromatic bridging ligands w...Date2023.05.08 Bywebmaster2 Views2075 -
Read More
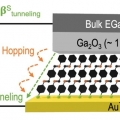
Thermopower in Transition from Tunneling to Hopping
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlThe Seebeck effect of molecular junction in a hopping regime or tunneling-to-hopping transition remains uncertain. This paper describes the Seebeck effect in molecular epitaxy films (OPIn where n = 1 – 9) based on imine condensation be...Date2023.05.08 Bywebmaster2 Views654 -
Read More
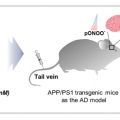
An Activity-Based Fluorescent Probe for Imaging Fluctuations of Peroxynitrite (ONOO-) in the Alzheimer's Disease Brain
Jong Seung Kimhttp://orgchem.korea.ac.kr/Peroxynitrite (ONOO-) plays a critical role in Alzheimer's disease (AD), and the association between ONOO- and AD is inexplicit. To reveal the ONOO- influxes in AD brains, an activatable activity-based fluorescence probe Rd-DPA3 was rati...Date2023.05.08 Bywebmaster2 Views730 -
Read More
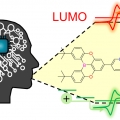
Deep learning for development of organic optoelectronic devices: Efficient prescreening of hosts and emitters in deep-blue fluorescent OLEDS
Dong Hoon Choi and Sungnam Parkhttps://ultrafastspec.wixsite.com/sparkThe highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies, which are key factors in optoelectronic devices, must be accurately estimated for newly designed materials. Here, we developed a deep lear...Date2023.05.08 Bywebmaster2 Views918 -
Read More
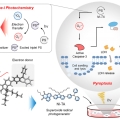
Photocatalytic Superoxide Radical Generator that Induces Pytoptosis in Cancer Cells
Jong Seung Kimhttp://orgchem.korea.ac.kr/In this study, we show that a photocatalytic superoxide radical (O2−•) generator, NI-TA, triggers pyroptosis in cancer cells. NI-TA was designed to take advantage of an intramolecular triplet-ground state splitting energy modulati...Date2023.05.08 Bywebmaster2 Views683 -
Read More
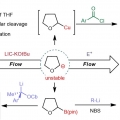
Direct C-H metallation of tetrahydrofuran and application in flow
Heejin Kimhttps://gowithflow.wixsite.com/hkimThe direct C–H metallation of tetrahydrofuran (THF) to generate α-anionic THF is one of the most straightforward methods for the the generation and utilization of α-anionic THF. Here we develop a reaction for the direct met...Date2023.05.08 Bywebmaster2 Views953 -
Read More
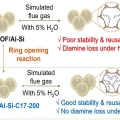
Functionalization of Diamine-Appended MOF-Based Adsorbents by Ring Opening of Epoxide: Long-Term Stability and CO2 Recyclability under Humid Conditions
Chang Seop Honghttps://www.immlab.korea.ac.kr/Although diamine-appended metal-organic framework (MOF) adsorbents exhibit excellent CO2 adsorption performance, a continuous decrease in long-term capacity during repeated wet cycles remains a formidable challenge for practical applications...Date2023.05.08 Bywebmaster2 Views737 -
Read More
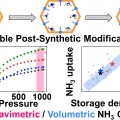
High Gravimetric and Volumetric Ammonia Capacities in Robust Metal-Organic Frameworks Prepared via Double Postsynthetic Modification
Chang Seop Honghttps://www.immlab.korea.ac.kr/Ammonia is a promising energy vector that can store the high energy density of hydrogen. For this reason, numerous adsorbents have been investigated as ammonia storage materials, but ammonia adsorbents with a high gravimetric/volumetric ammo...Date2023.05.08 Bywebmaster2 Views1079 -
Read More
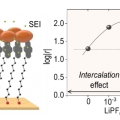
Li-ion Intercalation, Rectification, and Solid Electrolyte Interphase in Molecular Tunnel Junctions
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlThis paper describes Li-ion intercalation into pyrenyl terminated self-assembled monolayer (SAM) on gold, inspired from graphite anode in Li-ion battery, and its effect on tunneling performance in molecular junction incorporating the SAM. As...Date2023.05.08 Bywebmaster2 Views563 -
Read More
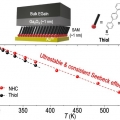
Thermopower of Molecular Junction in Harsh Thermal Environments
Hyo Jae Yoonhttps://hyojaeyoon.wixsite.com/ommlMolecular junctions can be miniaturized devices for heat-to-electricity conversion application, yet these operate only in mild thermal environments (less than 323 K) because thiol, the most widely used anchor moiety for chemisorption of acti...Date2023.05.08 Bywebmaster2 Views490 -
Read More

Microfluidics-Assisted Synthesis of Hierarchical Cu2O Nanocrystal as C2-Selective CO2 Reduction Electrocatalyst
Heejin Kim, Kwangyeol Leehttp://nanolab.korea.ac.kr/Copper-based catalysts have attracted enormous attention due to their high selectivity for C2+ products during the electrochemical reduction of CO2 (CO2RR). In particular, grain boundaries on the catalysts contribute to the generation of var...Date2023.05.08 Bywebmaster2 Views1297
Designed by sketchbooks.co.kr / sketchbook5 board skin