
Adsorption of Carbon Dioxide on Unsaturated Metal Sites in M2(dobpdc) Frameworks with Exceptional Structural Stability and Relation between Lewis Acidity and Adsorption Enthalpy
| author | Chang Seop Hong |
|---|---|
| Homepage | http://immlab.korea.ac.kr/ |
| journal | Chem. Eur. J. 2016, 22,7444 –7451 |
| Attachment '1' |
|---|
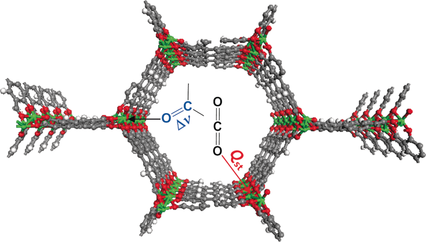
A series of metal–organic frameworks (MOFs) M2(dobpdc) (M=Mn, Co, Ni, Zn; H4dobpdc=4,4′-dihydroxy-1,1′-biphenyl-3,3′-dicarboxylic acid), with a highly dense arrangement of open metal sites along hexagonal channels were prepared by microwave-assisted or simple solvothermal reactions. The activated materials were structurally expanded when guest molecules including CO2 were introduced into the pores. The Lewis acidity of the open metal sites varied in the order Mn<Co<Ni>Zn, as confirmed by C=O stretching bands in the IR spectra, which are related to the CO2 adsorption enthalpy. DFT calculations revealed that the high CO2 binding affinity of transition-metal-based M2(dobpdc) is primarily attributable to the favorable charge transfer from CO2(oxygen lone pair acting as a Lewis base) to the open metal sites (Lewis acid), while electrostatic effects, the underlying factor responsible for the particular order of binding strength observed across different transition metals, also play a role. The framework stability against water coincides with the order of Lewis acidity. In this series of MOFs, the structural stability of Ni2(dobpdc) is exceptional; it endured in water vapor, liquid water, and in refluxing water for one month, and the solid remained intact on exposure to solutions of pH 2–13. The DFT calculations also support the experimental finding that Ni2(dobpdc) has higher chemical stability than the other frameworks.
http://onlinelibrary.wiley.com/doi/10.1002/chem.201600189/full
« Prev Investigation of Charge Carrier Behavior in High Performance ...
 Investigation of Charge Carrier Behavior in High Performance ...
2016.07.29by Manager
〈
Investigation of Charge Carrier Behavior in High Performance ...
2016.07.29by Manager
〈
Programmed activation of cancer cell apoptosis: A tumor-targe... Next »
 Programmed activation of cancer cell apoptosis: A tumor-targe...
2016.07.29by Manager
〉
Programmed activation of cancer cell apoptosis: A tumor-targe...
2016.07.29by Manager
〉
-
Read More
Highly Efficient Fullerene-Free Polymer Solar Cells Fabricated with Polythiophene Derivative
Han Young Woohttp://www.ooml.korea.ac.kr/A highly efficient fullerene-free polymer solar cell (PSC) based on PDCBT, a polythiophene derivative substituted with alkoxycarbonyl, has achieved an impressive power conversion efficiency of 10.16%, which is the best result in PSCs based o...Date2016.09.29 ByManager Views3621 -
Read More
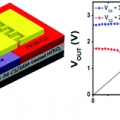
Low-voltage organic devices based on pristine and self-assembled monolayer-treated HfTiOx gate dielectrics
Jong-Ho Choihttp://neslab.campushomepage.com/index2.aspLow-voltage organic field-effect transistors (OFETs) and complementary metal oxide semiconductor (CMOS) inverters based on pentacene and N,N′-ditridecylperylene-3,4,9,10-tetracarboxylic diimide (PTCDI-C13) were fabricated on HfTiOx gat...Date2016.09.20 ByManager Views1670 -
Read More
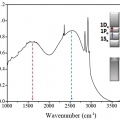
Higher Quantum State Transitions in Colloidal Quantum Dot with Heavy Electron Doping
Kwang Seob Jeonghttps://sites.google.com/site/ksjkulab/Electron occupation in the lowest quantized state of the conduction band (1Se) in the colloidal quantum dot leads to the intraband transition in steady-state (1Se-1Pe). The intraband transition, solely originating from the quantum confinemen...Date2016.09.20 ByManager Views3348 -
Read More
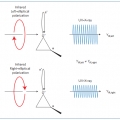
High-harmonic generation: Drive round the twist
Minhaeng Chohttp://cmsd.ibs.re.kr/html/cmsd_en/Light has long been used to detect the chirality of molecules but high-order harmonic generation now provides access to these chiral interactions on ultrafast timescales. http://www.nature.com/nphys/journal/v11/n8/full/nphys3395.htmlDate2016.09.20 ByManager Views1741 -
Read More
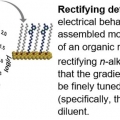
Gradients of Rectification: Tuning Molecular Electronic Devices by the Controlled Use of Different-Sized Diluents in Heterogeneous Self-Assembled Monolayers
Hyo Jae YoonMolecular electronics has received significant attention in the last decades. To hone performance of devices, eliminating structural defects in molecular components inside devices is usually needed. We herein demonstrate this problem can be ...Date2016.07.29 ByManager Views1938 -
Read More
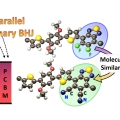
Investigation of Charge Carrier Behavior in High Performance Ternary Blend Polymer Solar Cells
Han Young Woohttp://www.ooml.korea.ac.kr/This study demonstrates high-performance, ternary-blend polymer solar cells by modifying a binary blend bulk heterojunction (PPDT2FBT:PC71BM) with the addition of a ternary component, PPDT2CNBT. PPDT2CNBT is designed to have complementary ab...Date2016.07.29 ByManager Views1825 -
Read More
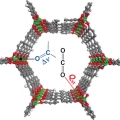
Adsorption of Carbon Dioxide on Unsaturated Metal Sites in M2(dobpdc) Frameworks with Exceptional Structural Stability and Relation between Lewis Acidity and Adsorption Enthalpy
Chang Seop Honghttp://immlab.korea.ac.kr/A series of metal–organic frameworks (MOFs) M2(dobpdc) (M=Mn, Co, Ni, Zn; H4dobpdc=4,4′-dihydroxy-1,1′-biphenyl-3,3′-dicarboxylic acid), with a highly dense arrangement of open metal sites along hexagonal channels wer...Date2016.07.29 ByManager Views5563 -
Read More
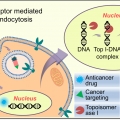
Programmed activation of cancer cell apoptosis: A tumor-targeted phototherapeutic topoisomerase I inhibitor
Jong Seung Kimhttp://orgchem.korea.ac.kr/index2.aspWe report here a tumor-targeting masked phototherapeutic agent 1 (PT-1). This system contains SN-38—a prodrug of the topoisomerase I inhibitor irinotecan. Topoisomerase I is a vital enzyme that controls DNA topology during replication,...Date2016.07.29 ByManager Views12887 -
Read More
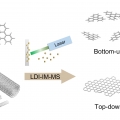
Probing Distinct Fullerene Formation Processes from Carbon Precursors of Different Sizes and Structures
Hugh I Kimhttps://sites.google.com/site/hughkimgroup/Fullerenes, cage-structured carbon allotropes, have been the subject of extensive research as new materials for diverse purposes. Yet, their formation process is still not clearly understood at the molecular level. In this study, we performe...Date2016.07.29 ByManager Views3860 -
Read More
Manifesting Subtle Differences of Neutral Hydrophilic Guest Isomers in a Molecular Container by Phase Transfer
Hugh I. Kimhttps://sites.google.com/site/hughkimgroup/Achieving strong host–guest interactions between synthetic hosts and hydrophilic guests in solution is challenging because solvation effects overwhelm other effects. To resolve this issue, we transferred complexes of cucurbit[7]uril (C...Date2016.07.29 ByManager Views1622
Designed by sketchbooks.co.kr / sketchbook5 board skin