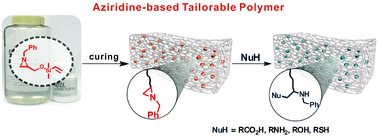
Aziridine-Functionalized Polydimethylsiloxanes for Tailorable Polymeric Scaffolds: Aziridine as a Clickable Moiety for Structural Modification of Materials
| author | Hyo Jae Yoon |
|---|---|
| Homepage | https://sites.google.com/site/ommlatku/ |
| journal | Polym. Chem., 2017(DOI:10.1039/C7PY00317J) |
| Attachment '1' |
|---|
This paper shows that tailorable polymeric scaffolds on a molecular scale could be achieved through the ring opening reaction of a three-membered N-heterocyclic compound, aziridine. Aziridine is incorporated into an elastomeric polymer backbone (here, PDMS) through Pt(0)-catalyzed hydrosilylation, retaining attractive features such as optical transparency and elastic properties compared to conventional PDMS. The resulting aziridine-containing PDMS is chemoselectively and regio-specifically post-modified through an orthogonal ring opening reaction of the aziridine and takes advantage of the wide substrate scope of aziridine chemistry.
http://pubs.rsc.org/en/content/articlelanding/2017/py/c7py00317j#!divAbstract
« Prev Excellent Long-Term Stability of Power Conversion Efficiency...
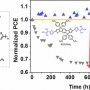 Excellent Long-Term Stability of Power Conversion Efficiency...
2017.04.06by Manager
〈
Excellent Long-Term Stability of Power Conversion Efficiency...
2017.04.06by Manager
〈
Effect of ion-ligand binding on ion pairing dynamics studied ... Next »
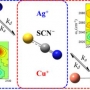 Effect of ion-ligand binding on ion pairing dynamics studied ...
2017.04.06by Manager
〉
Effect of ion-ligand binding on ion pairing dynamics studied ...
2017.04.06by Manager
〉
-
Read More
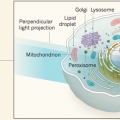
Cell imaging: An intracellular dance visualized
Sang-Hee Shimhttp://Nature 546, 39–40 (doi:10.1038/nature22500)The development of a microscopy technique that enables observation of the interactions between six types of organelle, in 3D and over time, holds promise for improving our understanding of intracellular processes. See Letter p.162 http://ww...Date2017.06.27 ByManager Views3903 -
Read More
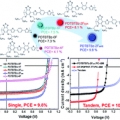
High-efficiency photovoltaic cells with wide optical band gap polymers based on fluorinated phenylene-alkoxybenzothiadiazole
Han Young Woohttp://www.ooml.korea.ac.kr/A series of semi-crystalline, wide band gap (WBG) photovoltaic polymers were synthesized with varying number and topology of fluorine substituents. To decrease intramolecular charge transfer and to modulate the resulting band gap of D–...Date2017.06.12 ByManager Views1637 -
Read More
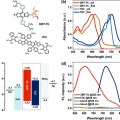
Eco-Friendly Solvent-Processed Fullerene-Free Polymer Solar Cells with over 9.7% Efficiency and Long-Term Performance Stability
Dong Hoon Choihttp://fpl.korea.ac.kr/index2.aspA wide-bandgap polymer, (poly[(2,6-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-(2,5-(methyl thiophene carboxylate))]) (3MT-Th), is synthesized to obtain a complementary broad range absorption when harmo...Date2017.06.12 ByManager Views4115 -
Read More

Iridium-Based Multimetallic Nanoframe@Nanoframe Structure: An Efficient and Robust Electrocatalyst toward Oxygen Evolution Reaction
Kwangyeol Leehttp://nanolab.korea.ac.kr/Nanoframe electrocatalysts have attracted a great interest due to their inherently high active surface area per a given mass. Although recent progress has enabled the preparation of single nanoframe structures with a variety of morphologies,...Date2017.06.12 ByManager Views1362 -
Read More
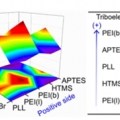
Formation of Triboelectric Series via Atomic Level Surface Functionalization for Triboelectric Energy Harvesting
Hyo Jae Yoonhttp://sites.google.com/site/ommlatkuTriboelectric charging involves frictional contact of two different materials, and their contact electrification usually relies on polarity difference in the triboelectric series. This limits the choices of materials for triboelectric contac...Date2017.05.31 ByManager Views4169 -
Read More
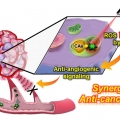
Overcoming the Limits of Hypoxia in Photodynamic Therapy: A Carbonic Anhydrase IX-Targeted Approach
Jong Seung Kimhttp://orgchem.korea.ac.kr/index2.aspA major challenge in photodynamic cancer therapy (PDT) is avoiding PDT-induced hypoxia, which can lead to cancer recurrence and progression through activation of various angiogenic factors and significantly reduce treatment outcomes. Reporte...Date2017.05.10 ByManager Views2639 -
Read More
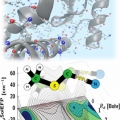
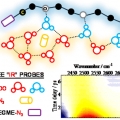
Vibrational Probes: From Small Molecule Solvatochromism Theory and Experiments to Applications in Complex Systems
Minhaeng Chohttp://cmsd.ibs.re.kr/html/cmsd_en/The vibrational frequency of a chosen normal mode is one of the most accurately measurable spectroscopic properties of molecules in condensed phases. Accordingly, infrared absorption and Raman scattering spectroscopy have provided valuable ...Date2017.04.26 ByManager Views1866 -
Read More
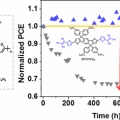
Excellent Long-Term Stability of Power Conversion Efficiency in Non-Fullerene-Based Polymer Solar Cells Bearing Tricyanovinylene-Functionalized n-Type Small Molecules
Dong Hoon Choihttp://fpl.korea.ac.kr/index2.aspNew small molecules having modified acceptor strength and π-conjugation length and containing dicyanovinylene (DCV) and tricyanovinylene (TCV) as a strongly electron-accepting unit with indacenodithiophene, IDT(DCV)2, IDT(TCV)2, and IDTT(...Date2017.04.06 ByManager Views11061 -
Read More
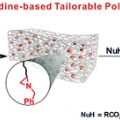
Aziridine-Functionalized Polydimethylsiloxanes for Tailorable Polymeric Scaffolds: Aziridine as a Clickable Moiety for Structural Modification of Materials
Hyo Jae Yoonhttps://sites.google.com/site/ommlatku/This paper shows that tailorable polymeric scaffolds on a molecular scale could be achieved through the ring opening reaction of a three-membered N-heterocyclic compound, aziridine. Aziridine is incorporated into an elastomeric polymer backb...Date2017.04.06 ByManager Views1987 -
Read More
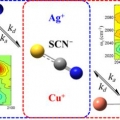
Effect of ion-ligand binding on ion pairing dynamics studied by two-dimensional infrared spectroscopy
Sungnam Parkhttp://ultrafastspec.wixsite.com/sparkCation-specific ion pairing dynamics between M+ (M=Ag or Cu) and SCN−in N,N-dimethylthioformamide (DMTF) are studied by vibrationally probing the nitrile (CN) stretching vibration. SCN− ion, which is an ambidentate ligand, readil...Date2017.04.06 ByManager Views1908 -
Read More
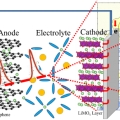
Ultrafast fluxional exchange dynamics in electrolyte solvation sheath of lithium ion battery
Kyungwon Kwak & Minhaeng Chohttp://cmsd.ibs.re.kr/html/cmsd_en/Lithium cation is the charge carrier in lithium-ion battery. Electrolyte solution in lithium-ion battery is usually based on mixed solvents consisting of polar carbonates with different aliphatic chains. Despite various experimental evidence...Date2017.03.31 ByManager Views1879 -
Read More
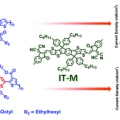
Molecular design of a wide-band-gap conjugated polymer for efficient fullerene-free polymer solar cells
Han Young Woohttp://www.ooml.korea.ac.kr/Two p-type conjugated polymers with disparate optical and electronic properties, PB3T and PB2T, were developed and applied in fullerene-free polymer solar cells (PSCs). The photovoltaic performance of the PB3T-based PSC device processed by a...Date2017.03.31 ByManager Views1885 -
Read More

Multiplexed Post-Experimental Monoisotopic Mass Refinement (mPE-MMR) to Increase Sensitivity and Accuracy in Peptide Identifications from Tandem Mass Spectra of Cofragmentation
Sang-Won Leehttp://ionchem.korea.ac.kr/Mass spectrometry (MS)-based proteomics, which uses high-resolution hybrid mass spectrometers such as the quadrupole-orbitrap mass spectrometer, can yield tens of thousands of tandem mass (MS/MS) spectra of high resolution during a routine b...Date2017.03.31 ByManager Views4033 -
Read More
Key Structural Elements for Cellular Uptake of Acinetobactin, a Major Siderophore of Acinetobacter baumannii
Hak Joong Kimhttps://sites.google.com/site/kubccb/Acinetobactin is a major siderophore utilized by the human pathogen Acinetobacter baumannii. The rapid acquisition of drug resistance by A. baumannii has garnered concern globally. Herein, acinetobactin and systematically generated analogues...Date2017.02.07 ByManager Views3287 -
Read More
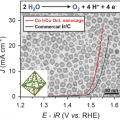
Cobalt Assisted Synthesis of IrCu Hollow Octahedral Nanocages as Highly Active Electrocatalysts toward Oxygen Evolution Reaction
Kwangyeol Leehttp://nanolab.korea.ac.kr/Development of oxygen evolution reaction (OER) catalysts with reduced precious metal content while enhancing catalytic performance has been of pivotal importance in cost-effective design of acid polymer electrolyte membrane water electrolyze...Date2017.02.07 ByManager Views1585 -
Read More
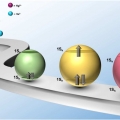
Singly and Doubly Occupied Higher Quantum State in Nanocrystals
Kwang Seob Jeonghttps://sites.google.com/site/ksjkulab/Filling the lowest quantum state of the conduction band (CB) of colloidal nanocrystals with a single electron, which is analogous to the filling the lowest unoccupied molecular orbital (LUMO) in a molecule with a single electron, has attract...Date2017.01.24 ByManager Views1848 -
Read More
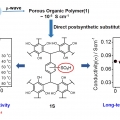
Cost-Effective, High-Performance Porous-Organic-Polymer Conductors Functionalized with Sulfonic Acid Groups by Direct Postsynthetic Substitution
Chang Seop Honghttp://immlab.korea.ac.kr/We demonstrate the facile microwave-assisted synthesis of a porous organic framework 1 and the sulfonated solid (1S) through postsubstitution. Remarkably, the conductivity of 1S showed an approximately 300-fold enhancement at 30 °...Date2016.12.22 ByManager Views1976 -
Read More
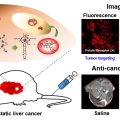
Liposomal Texaphyrin Theranostics for Metastatic Liver Cancer
Jong Seung Kimhttp://orgchem.korea.ac.kr/index2.aspReported here is a new theranostic agent, 1, which consists of a Gd3+-texaphyrin core conjugated to a doxorubicin prodrug via a disulfide bond. Conjugate 1 was designed to undergo cleavage in the presence of glutathione (GSH), a species typi...Date2016.12.21 ByManager Views2986 -
Read More
Water Dynamics in Cytoplasm-Like Crowded Environment Correlates with the Conformational Transition of the Macromolecular Crowder
Minhaeng Chohttp://cmsd.ibs.re.kr/html/cmsd_en/Polyethylene glycol (PEG) is a unique polymer material with enormous applicability in many industrial and scientific fields. Here, its use as macromolecular crowder to mimic the cellular environment in vitro is the focus of the present study...Date2016.12.16 ByManager Views6051 -
Read More
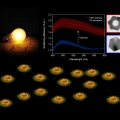
Plasmon Enhanced Direct Bandgap Emissions in Cu7S4@Au2S@Au Nanorings
Kwangyeol Leehttp://nanolab.korea.ac.kr/Nanostructured copper sulfides, promising earth-abundant p-type semiconductors, have found applications in a wide range of fields due to their versatility, tunable low bandgap, and environmental sustainability. The synthesis of hexagonal Cu7...Date2016.09.29 ByManager Views1961
Designed by sketchbooks.co.kr / sketchbook5 board skin