[Thu. 26 Oct. 2017] Dr. Jung-Nyoung Heo/Center for Medicinal Chemistry Research, Korea Research Institute of Chemical Technology, Daejeon, Korea
| Speaker | Dr. Jung-Nyoung Heo |
|---|---|
| Date | Thu. 26 Oct. 2017 |
| Time | 5:00pm |
| Venue | #331, Asan Hall, College of Science |
“Journey to the Discovery of Anticancer Drugs and
Synthetic Studies on Polycyclic Heterocycles”
In an area of organic synthesis and medicinal chemistry, exploration of practical and efficient synthetic methods provides great interests to the search of bioactive compounds, natural products, and pharmaceutical agents. In recent years, our laboratory have been particularly interested in the development of new one-pot synthetic approaches for rapid, facile, and economic construction of a number of biologically interesting natural products. During this process, research efforts from our group have focused on the discovery of new anticancer agents with specific molecular targets. In particular, we have discovered STP06-1002 as a potent, first-in-class tankyrase inhibitor that expresses anticancer activity against colon cancer.
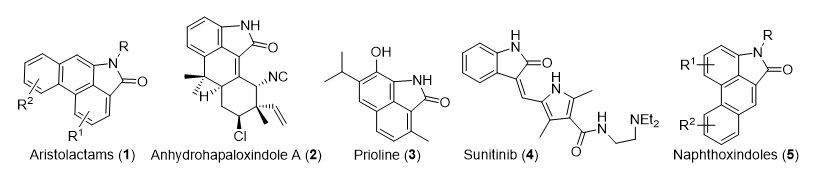
Polycyclic compounds possessing an oxindole moiety are important in the search for novel, biologically active agents for the pharmaceutical and agrochemical industries. As part of our ongoing efforts to develop structurally unique motifs for potential use in pharmaceuticals, we focused on the natural products derived from 2-oxoindoles (Figure 1). Naturally occurring oxindoles include aristolactams (1), anhydrohapaloxindole A (2), and prioline (3). The large family of aristolactams (1) exhibits various bioactivities, including antitumor effects against human cancer cell lines. Anhydrohapaloxindole A (2) was isolated from a cultured strain of a terrestrial blue-green alga. Prioline (3) was isolated from the roots of a species of Salvia, a genus whose plants are used in Chinese folk medicine for the treatment of tonsillitis, pharyngitis, pulmonary tuberculosis, and bacillary dysentery. Of particular interest is sunitinib (4), which is a highly active receptor tyrosine kinase inhibitor used in the treatment of advanced renal cell carcinoma and gastrointestinal stromal tumours. In this seminar, our efforts towards the syntheses of naphthoxindoles (5)1 and naphthostyrils including natural prioline (3)2 will be presented.

 Figure 1. Natural and medicinal compounds possessing an oxindole moiety.
Figure 1. Natural and medicinal compounds possessing an oxindole moiety.
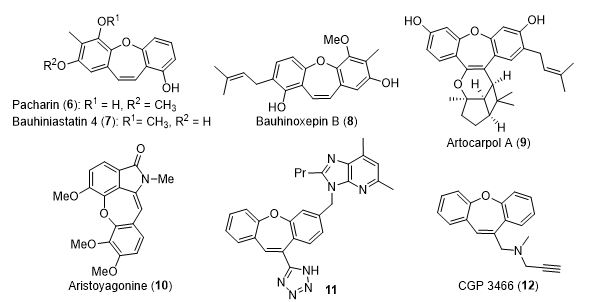
In addition, dibenzo[b,f]oxepin is an important motif in natural and medicinal compounds (Figure 2). Recently, pacharin (6) and bauhiniastatins 1-4 (7) were isolated from the plant Bauhinia purpurea, and these compounds were shown to significantly inhibit cancer cell growth. These compounds are similar to the natural products bauhinoxepin B (8) and artocarpol A (9), which have been shown to exhibit antimycobacterial and anti-inflammatory activities, respectively. Additionally, aristoyagonine (10) occupies a special interest since it is the only example to date of a natural cularine alkaloid incorporating a five-membered lactam. Due to their low natural occurrence in conjunction with their biological activity against various cancer cell lines, several synthetic tactics have been disclosed for the synthesis of these structurally unusual alkaloids. Notably, molecules containing the dibenzo[b,f]oxepin moiety have received considerable interest from the medicinal community due to these compounds’ potent biological properties, such as antipsychotic, antidepressant, antihypertensive, antiestrogenic, anti-inflammatory, and insecticidal activities. For example, compound 11 is a nonpeptide angiotensin II receptor antagonist that can regulate blood pressure and electrolyte homeostasis. Additionally, CGP 3466 (12) exhibits strong neuroprotective activity as the result of its ability to prevent neuronal apoptosis in the adult brain.3
Figure 2. Dibenzo[b,f]oxepins in natural and medicinal compounds.
Previously, we developed a strategy for the direct one-pot synthesis of phenanthrenes that employs a Suzuki-Miyaura coupling/aldol condensation cascade sequence.4 Then, we reported the application of this procedure to the total synthesis of aristolactams, including aristolactam BII, aristolactam BIII, aristolactam FI,N-methyl piperolactam A, and sauristolactam.5 As part of a research program to develop a one-pot metal-catalyzed reaction/aldol condensation reaction, we sought to develop an efficient synthesis of dibenzo[b,f]oxepin via a one-pot Cu-catalyzed aryl ether formation/aldol condensation reaction.6 Herein, we will discuss the synthetic methodologies for the construction of diverse polycyclic heterocyclic scaffolds via an efficient one-pot procedure.
References
1. Park, K.-Y.; Kim, B. T.; Heo, J.-N. Eur. J. Org. Chem. 2014, 164.
2. Park, K.-Y.; Song, H.-J.; Heo, J.-N. Adv. Synth. Catal. 2015, 357, 3197.
3. (a) Zimmermann, K.; Waldmeier, P. C.; Tatton, W. G. Pure Appl. Chem. 1999, 71, 2039–2046. (b) Zimmermann, K.; Roggo, S.; Kragten, E.; F€urst, P.; Waldmeier, P. Bioorg. Med. Chem. Lett. 1998, 8, 1195–1200. (c) Sagot, Y.; Toni, N.; Perrelet, D.; Lurot, S.; King, B; Rixner, H.; Mattenberger, L.; Waldmeier, P. C.; Kato, A. C. Br. J. Pharmacol. 2000, 131, 721–728.
4. Kim, Y. H.; Lee, H.; Kim, Y, J.; Kim, B. T.; Heo, J.-N. J. Org. Chem. 2008, 73, 495–501.
5. Kim, J. K.; Kim, Y. H.; Nam, H, T.; Kim, B. T.; Heo, J.-N. Org. Lett. 2008, 10, 3543–3546.
6. (a) Choi, Y. L.; Lim, H. S.; Heo, J.-N. Org. Lett. 2012, 14, 5102. (b) Lim, H. S.; Choi, Y. L.; Heo, J.-N. Org. Lett. 2013, 15, 4718.
-
Read More
Fall semester 2016 Seminar Schedule
-- -
Read More
Spring semester 2017 Seminar Schedule
-- -
Read More
[Fri. 17 Mar. 2017] 9th RINS Symposium
-Fri. 17 Mar. 2017 -
Read More
[Thu. 27 Apr. 2017] Seminar was canceled!
-- -
Read More
[Wed. 5 July. 2017] Seminar is canceled!
-- -
Read More
7th Jilin-Korea-Waseda Alliance Annual Symposium
-August 17 ~ 19, 2017 -
Read More
Fall semester 2017 Seminar Schedule
-- -
Read More
Fall semester 2018 Seminar Schedule
-- -
Read More
Spring semester 2019 Seminar Schedule
-- -
Read More
Announcements Graduate Seminar Spring Semester 2021
.. -
Read More
Announcement for Graduate Seminar Class, Fall Semester 2020
Announcement for Graduate Seminar Class, Fall Semester 2020Announcement for Graduate Seminar Class, Fall Semester 2020 -
Read More
[October 31. 2019] Engineering energy band structures in organic and hybrid semiconductor devices using non-conjugated polyelectrolyte films
Asst. Prof. Bright Walker(Kyung Hee University)Thu. 31 October. 2019 -
Read More
June 10th (Fri) Graduate Seminar / Bing Zhang, Ph.D.
Bing Zhang, Ph.D. (Baylor College of Medicine)Fri, Jun 10, 2022 -
Read More
2022 International Symposium on Bioactive and Bioengineering Materials
BK21 Chemistry Education Research CenterNovember 22, 2022 (Tuesday) -
Read More
2022 International Symposium on Chemical Applications of Machine Learning
BK21 Chemistry Education Research CenterNovember 11, 2022 (Fri) -
Read More
2023 Germany-Korea On-Site Plenary Discussion on Computational Electrochemistry
BK21 Chemistry Education Research CenterApril 17, 2023 (Mon) -
Read More
[October 10, 2017] Novel Terahertz Spectroscopies
Director Mischa BonnTue, October 10, 2017 -
Read More
[August 14. 2019] Principle of Semiconductor Quantum Cascade Laser and It’s Application to Chemical Gas Detection
Dr. Byeong-il Seo(Agency for Defense Development)Wed. 14 August. 2019 -
Read More
[October 25, 2017] Ultrafast electron microscopy: applications in chemical and materials sciences
Dr. Byung-Kuk YooOctober 25, 2017 -
Read More
May 11, 2023 (Thu) Dr. Changyeol Lee
Dr. Changyeol Lee (Advanced Photonics Research Institute GIST)Thursday, May 11, 2023
Designed by sketchbooks.co.kr / sketchbook5 board skin